Method for synthesizing chlorambucil
A technology of chlorambucil and chlorambucil, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of low product yield, complicated post-processing purification process, many synthesis steps and the like , to achieve the effect of simple reaction and post-processing purification process, high reaction region selectivity and yield, and high anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
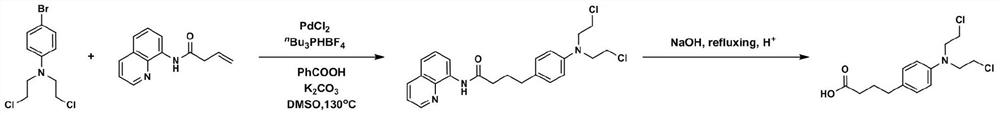
[0029] (1) Under an inert atmosphere, 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinoline-8- base) but-3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide in a molar volume ratio of 0.1mmol: 2mL was added to Mix in the reaction vessel;
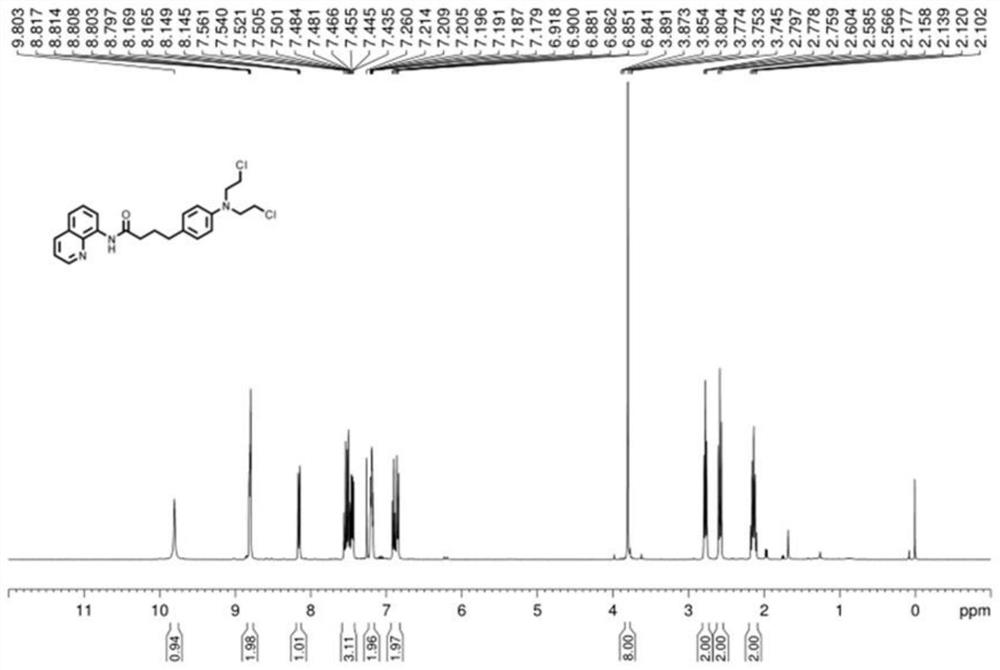
[0030] (2) Place the reaction vessel in an oil bath at 135°C and stir vigorously for 24 hours. The reaction product is washed with petroleum ether and ethyl acetate at a ratio of 1:5 to separate and purify the chromatographic silica gel column to obtain a compound with a leaving group. Detection determined that the compound was 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(quinolin-8-yl)butyramide;
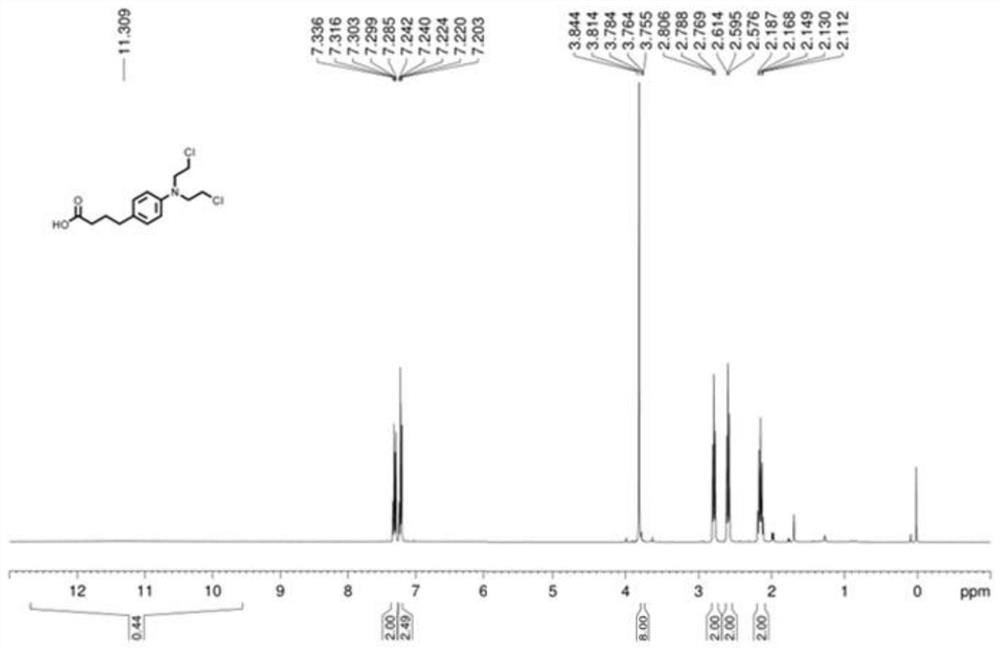
[0031] (3) After adding the compound capable of leaving the group into an aqueous solution of sodium hydroxide and heating to reflux for 12 hours, acidify to obtain chlorambucil, and the yield of chlorambucil is 41%.
Embodiment 2
[0033] (1) Under an inert atmosphere, 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinoline-8- base) but-3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide in a molar volume ratio of 0.1mmol: 2mL was added to Mix in the reaction vessel;
[0034] (2) Place the reaction vessel in an oil bath at 135°C and stir vigorously for 24 hours. The reaction product is washed with petroleum ether and ethyl acetate at a ratio of 1:5 to separate and purify the chromatographic silica gel column to obtain a compound with a leaving group. Detection confirmed that the compound was 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(quinolin-8-yl)butyramide.
[0035] (3) After adding the compound capable of leaving the group into an aqueous solution of sodium hydroxide and heating to reflux for 12 hours, acidify to obtain chlorambucil, and the yield of chlorambucil is 6%.
Embodiment 3
[0037] (1) Under an inert atmosphere, 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinoline-8- base) but-3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide in a molar volume ratio of 0.1mmol: 2mL was added to Mix in the reaction vessel;
[0038](2) The reaction vessel was placed in an oil bath at 135°C and stirred vigorously for 24 hours, and the reaction product was washed with petroleum ether and ethyl acetate at a ratio of 1:5 to separate and purify the chromatographic silica gel column to obtain a compound with a leaving group. Detection confirmed that the compound was 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(quinolin-8-yl)butyramide.
[0039] (3) After adding the compound capable of leaving the group into an aqueous solution of sodium hydroxide and heating to reflux for 12 hours, acidify to obtain chlorambucil, and the yield of chlorambucil is 9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com