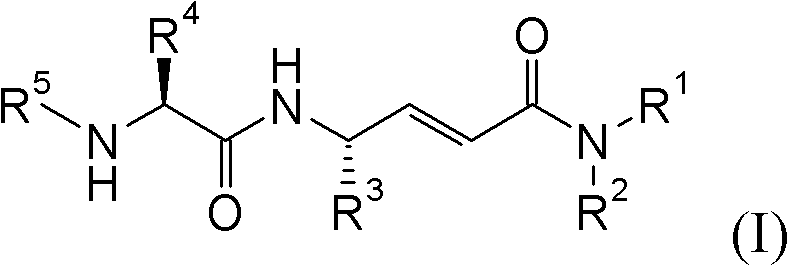
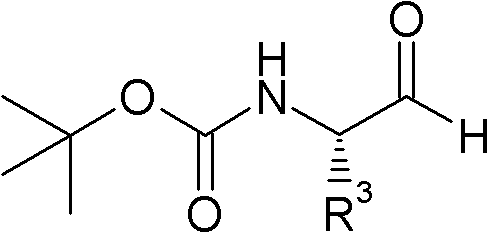
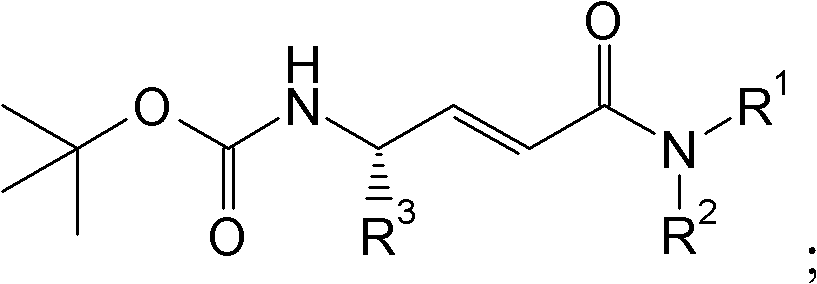
Cathepsin C inhibitors
A C1-C4, C1-C6 technology, applied in the field of 4-amino-2-butenamide compounds, can solve problems such as unverified processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0751] (2E,4S)-4-(L-alanylamino)-6-phenyl-N-(phenylmethyl)-2-hexenamide hydrochloride
[0752]
[0753] [(1S)-1-methyl-2-oxo-2-({(1S, 2E)-4-oxo-1-(2-phenylethyl)-4-[(phenylmethyl A solution of 1,1-dimethylethyl)amino]-2-buten-1-yl}amino)ethyl]carbamate (2.00 g, 4.3 mmol) in concentrated HCl (2.0 mL) was stirred at room temperature for 1 h . The reaction mixture was washed with saturated NaHCO 3 The aqueous solution was basified to pH 8 or 9, and then extracted with EtOAc (4x100 mL). The combined organic layers were washed with water (2x50 mL), washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo to give the free base of the title compound (0.60 g). The free base was dissolved in 1M HCl in Et 2 O solution (20 mL) was stirred for 2 h. The resulting solid was collected by filtration and washed with Et 2 O (10 mL) wash gave the title compound (0.30 g, 18%) as a white solid. LC-MS m / z 366(M+H) + , 1.59min (retention time).
Embodiment 2
[0755] (2E,4S)-4-(L-alanylamino)-N-methyl-6-phenyl-2-hexenamide hydrochloride
[0756]
[0757] To ((1S)-1-methyl-2-{[(1S,2E)-4-(methylamino)-4-oxo-1-(2-phenylethyl)-2-butene- CH 2 Cl 2 (30 mL) solution was added TFA (10 mL). The reaction mixture was stirred at room temperature for 2 h. The solvent was removed in vacuo, and Et was added 2 O (30 mL). The solid was filtered and washed with saturated NaHCO 3 Aqueous solution (20 mL), and then washed with water (10 mL) gave the free base of the title compound (400 mg). Dissolve the free base in 1M HCl in Et 2 O solution (20 mL) was stirred for 2 h. The resulting solid was collected by filtration and washed with Et 2 O (20 mL) washed to afford the title compound (0.28 g, 20%) as a white solid. LC-MS m / z 290(M+H) + , 1.34min (retention time).
Embodiment 3
[0759](2E,4S)-4-(L-alanylamino)-N,N-dimethyl-6-phenyl-2-hexenamide hydrochloride
[0760]
[0761] ((1S)-2-{[(1S,2E)-4-(dimethylamino)-4-oxo-1-(2-phenylethyl)-2-buten-1-yl] A solution of 1,1-dimethylethyl amino}-1-methyl-2-oxoethyl)carbamate (2.00 g, 4.96 mmol) in concentrated HCl (2.0 mL) was stirred at room temperature for 1 h. The reaction mixture was washed with saturated NaHCO 3 The aqueous solution was basified to pH 8 or 9, and then extracted with EtOAc (4x100 mL). The combined organic layers were washed with water (2x50 mL), washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo to give the free base of the title compound. Dissolve the free base in 1M HCl in Et 2 O solution (20 mL) was stirred for 2 h. The resulting solid was collected by filtration and washed with Et 2 O (10 mL) washed to afford the title compound (0.30 g, 20%) as a white solid. LC-MS m / z 304(M+H) + , 1.39min (retention time).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com