New use of quinazoline derivative tyrosine kinase inhibitor
A technology of uses and drugs, applied in the field of medicine, can solve problems such as uncontrolled cell reproduction and abnormality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
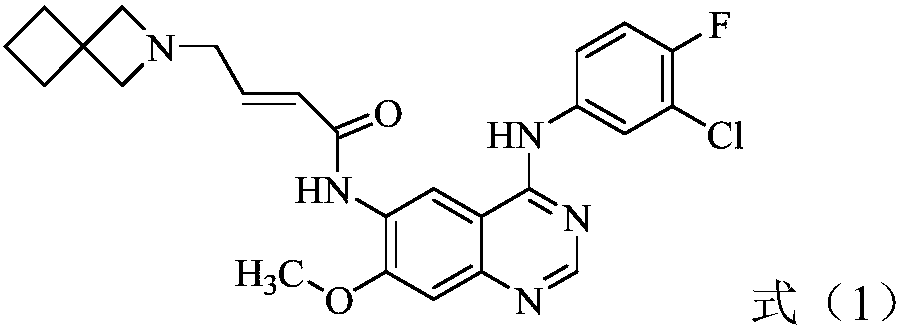
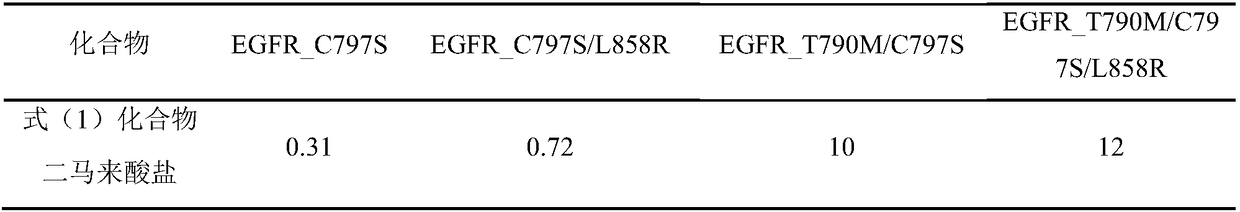
[0048] In vitro enzymatic activity test one of experimental example 1 formula (1) compound
[0049] Test product: dimaleate of the compound of formula (1), self-made.
[0050] Experimental method: Mobility Shift Assay was used to screen compounds on kinases EGFR_C797S, EGFR_C797S / L858R, EGFR_T790M / C797S and EGFR_T790M / C797S / L858R in the case of Km ATP.
[0051] 1. Reagent preparation
[0052] (1) 1x Kinase Buffer for Kinase Detection
[0053] 50mM HEPES (pH 7.5), 0.0015% Brij-35, 5mM MgCl 2 , 2mM DTT.
[0054] (2) Stop solution
[0055] 100 mM HEPES (pH 7.5), 0.015% Brij-35, 0.2% Coating Reagent #3, 50 mM EDTA.
[0056] 2. Compound preparation
[0057] (1) According to the highest concentration to be used, the compound was made into a 50× stock solution with 100% DMSO.
[0058] (2) Take a new 96-well plate, add 100 μL of compound master solution to the second well, and add 60 μL of 100% DMSO to the other wells. Take 20 μL of the compound from the second well and add i...
experiment example 2
[0082] In vitro enzymatic activity experiment two of experimental example 2 formula (1) compound
[0083] Test product: dimaleate of the compound of formula (1), self-made.
[0084] Experimental method: using the Mobility Shift Assay method in the case of Km ATP, in the kinase
[0085] Compounds were screened on EGFR_d746-750 / T790M / C797S.
[0086] 1. Reaction buffer
[0087] 20mM Hepes (pH 7.5), 10mM MgCl2, 1mM EGTA, 0.02% Brij35, 0.02mg / ml
[0088] BSA, 0.1mM Na 3 VO 4 , 2mM DTT, 1% DMSO.
[0089] 2. Compound preparation
[0090] (1) According to the highest concentration to be used, the compound was made into a 50× stock solution with 100% DMSO.
[0091] (2) Take a new 96-well plate, add 100 μL of compound master solution to the second well, and add 40 μL of 100% DMSO to the other wells. Take 20 μL of the compound from the second well and add it to the third well, and make 3-fold dilutions successively, and dilute 10 concentrations in total.
[0092](3) 100 μL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com