Substituted butenamide-N-oxide and preparation method and application thereof
A crotyl amide and oxide technology, applied in organic chemistry, drug combination, pharmaceutical formulations, etc., can solve the problems of low survival rate and poor patient prognosis, and achieve the effects of short reaction time, less three wastes, and environmentally friendly process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
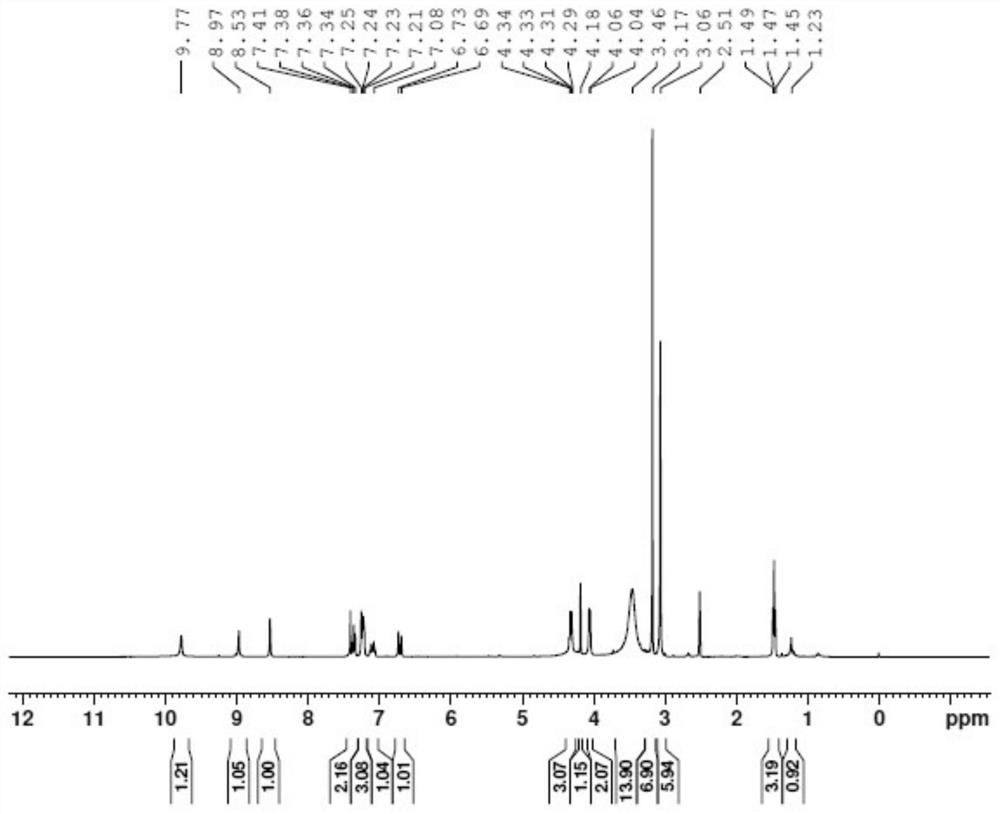
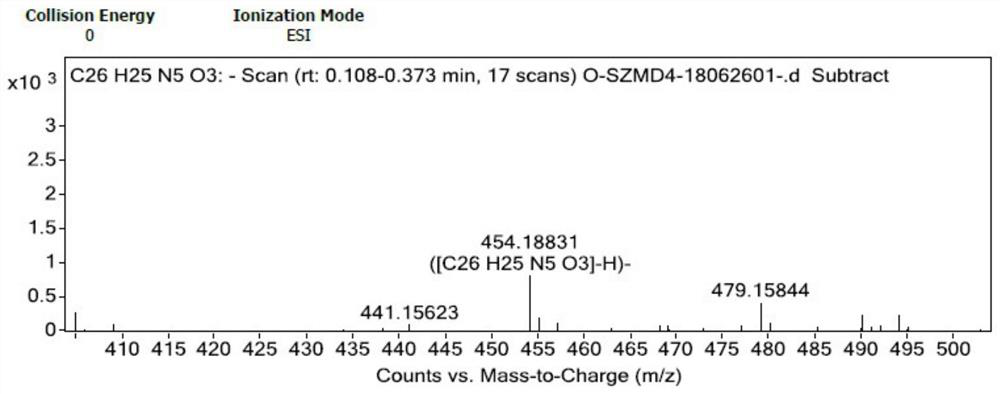
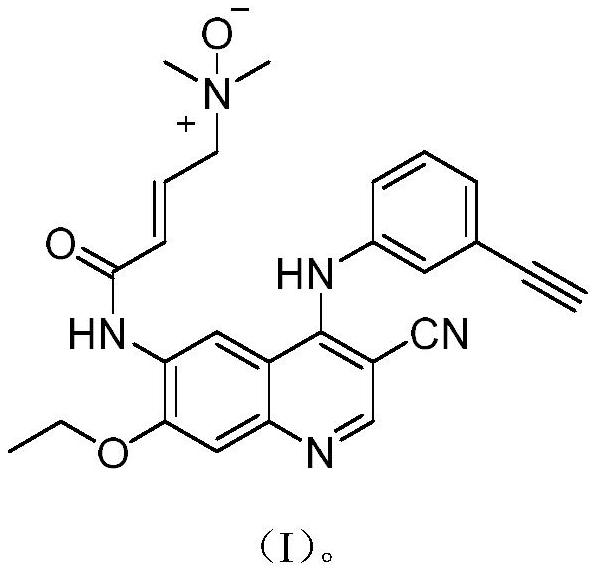
[0049] In a 100mL reaction flask, add 30mL of dichloromethane, and add 2.0g of (E)-N-[4-(3-ethynylphenyl)amino-3-cyano-7-ethoxyquinoline-6 -yl]-4-(dimethylamino)but-2-enamide (4.55mmol) was dissolved in dichloromethane, cooled to 0°C, and 2.0g (11.59mmol) of m-chloroperoxybenzoic acid was added, Stir the reaction for 20 minutes, after the reaction is complete as detected by TLC, add 20 mL of saturated sodium bicarbonate solution under stirring, a large amount of solids precipitate out, filter with suction, wash the filter cake with 10 mL of water, then wash with 10 mL of dichloromethane, and dry at 25°C for 24 hours to obtain (E )-N-[4-(3-ethynylphenyl)amino-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide N -Oxide 1.7g, yield 82.0%, moisture 13%, purity 98.87% (area normalization method).
[0050] To the obtained product (E)-N-[4-(3-ethynylphenyl)amino-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)butane- 2-enamide-N-oxide is detected, and its hydrogen spectr...
Embodiment 2
[0052] In a 250mL beaker, add 100mL dichloromethane, 50mL purified water, add 5.7g (E)-N-[4-(3-ethynylphenyl)amino-3-cyano-7-ethoxyquinoline- 6-yl]-4-(dimethylamino)but-2-enamide maleate (10mmol), stirred at room temperature, slowly added 5.5g of potassium carbonate, the solution gradually dissolved, left to stand, liquid separation , the aqueous phase was extracted with 20 mL of dichloromethane, combined with dichloromethane, washed with 30 mL of water, allowed to stand, separated, and dried overnight with 20.0 g of anhydrous sodium sulfate. Suction filtration, transfer the filtrate to a 250mL flask, cool down to -10°C, add m-chloroperoxybenzoic acid 2.0g (11.6mmol), stir below 0°C for 0.5h, naturally heat up, the reaction solution is white and turbid.
[0053] The reaction solution was poured into potassium carbonate 1.65g / 200mL aqueous solution, stirred for 10min, a large amount of white insoluble matter was filtered, washed with 300mL of water, beaten, filtered with suctio...
Embodiment 3
[0054] Embodiment 3: Drug efficacy test
[0055] 1. Purpose: To detect the ATP concentration when the compound of Example 1 inhibits four kinases to reach km by using Mobility shift assay; use staurosporine E as a positive control, the initial concentration is 10 μM, 4-fold dilution, 10 gradients , two parallel.
[0056] 2. Experimental materials:
[0057] EGFR (Camna, Cat.No 08-115, Lot.No 13CBS-0005M)
[0058] EGFR L858R (eurofins, Cat.No 14-626M, Lot.No 31001U)
[0059] EGFR (d746-750) (Carna, Cat.No 08-527, Lot.No 11CBS-1129F)
[0060] EGFR T790M (Invitrogen, Cat.No PV4804, Lot.No 1691293B)
[0061] Peptide FAM-P22 (GL Biochem, Cat.No.112393, Lot.No.P1801 16-MJ112393)
[0062] ATP (Sigma, Cat. No. A7699-1G, CAS No. 987-65-5)
[0063] DMSO (Sigma, Cat. No. D2650, Lot. No. 474382)
[0064] EDTA (Sigma, Cat. No. E5134, CAS No. 60-00-4)
[0065] 96-well plate (Coming, Cat.No.3365, Lot.No.22008026)
[0066]384-well plate (Cormning, Cat.No.3573, Lot.No.12608008)
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com