Application of 4-oxo-2-butenamide derivatives in the preparation of antibacterial agents
A technology of crotenamide and bacteriostatic agent, which is applied in the field of application of 4-oxo-2-butenamide derivatives in the preparation of bacteriostatic agents, and can solve problems such as threats and harm to human life and health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
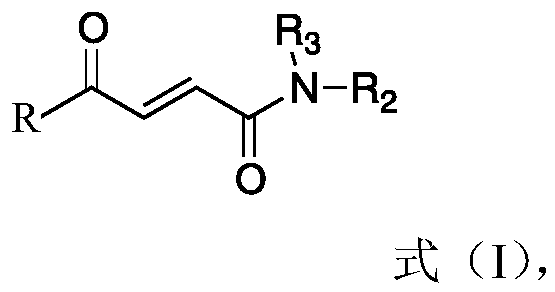
[0067] Specifically, the synthesis method may include the following steps:
[0068] (1) Formula (II) compound and glyoxylic acid generate formula (III) compound;
[0069] (2) the compound of formula (III) is condensed with an amine compound to obtain a compound of formula (I);
[0070] Wherein, in the step (1), the reaction condition is to use glacial acetic acid as the reaction solvent, and reflux reaction under the action of a catalytic amount of concentrated sulfuric acid to prepare the compound (III).
[0071] Wherein, in step (2), the reaction conditions are that compound (III) uses anhydrous dichloromethane as a solvent, first reacts with isobutyl chloroformate to generate a mixed anhydride, and then condenses with an amine compound to obtain compound (I); Or compound (III) is activated by carbodiimide condensing agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide with N,N-dimethylformamide as solvent, and then Condensation with amine compounds to obtain compound (I). ...
Embodiment 1
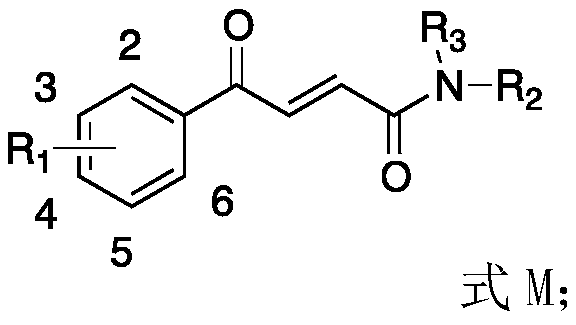
[0072] Embodiment 1, the synthesis of (E)-4-oxo-4-(1,1'-biphenyl)-2-butenamide (abbreviation I-1 or 4ph5) 1) β-bibenzoyl acrylic acid (abbreviation III-1) synthesis
[0073] In the 500mL reaction flask, add 4-biphenyl ethyl ketone (abbreviation II-1) (62.48g, 0.318mol), glyoxylic acid monohydrate (28.85g, 0.313mol), the acetic acid (250mL) of concentration 98% and concentration 98 % concentrated sulfuric acid (1mL), stirred, the material could not be completely dissolved, stirred and heated to 115 ° C, refluxed for about 0.5h, the reaction system was a yellow clear liquid, continued to react at the same temperature until the basic reaction of the raw materials was complete (TLC detection), after the reaction Evaporate acetic acid under reduced pressure, a large amount of white solid precipitated, add petroleum ether:ethyl acetate (volume ratio 2:1, 50mL), stir at room temperature for 0.5h, filter with suction, and dry under vacuum at 40°C to obtain the off-white solid product ...
Embodiment 2
[0079] Example 2, (E)-4-oxo-4-(1,1'-biphenyl)-N,N-dimethyl-2-butenamide (I-2) (referred to as 4ph5-2) Synthesis
[0080] Into the 200mL round bottom flask, add the β-bibenzoyl acrylic acid prepared in Example 1 (abbreviation III-1) (6.68g, 26.48mmol), anhydrous N,N-dimethylformamide (DMF, 30mL ), 1-hydroxybenzotriazole (HOBt, 4.29g, 31.77mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 10.15g, 52.96mmol ), electromagnetically stirred at room temperature to obtain a brown suspension A. Take another 25mL round bottom flask, add dimethylamine hydrochloride (3.0g, 36.79mmol), 5mL anhydrous DMF, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 2.59g, 31.78mmol), electromagnetically stirred at room temperature to obtain a colorless solution B. After 2h, the colorless solution B was added to the brown suspension A, followed by TLC, and the reaction was basically complete after 24h. It was quenched by adding 100 mL of water, extracted three times with 250 mL of di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com