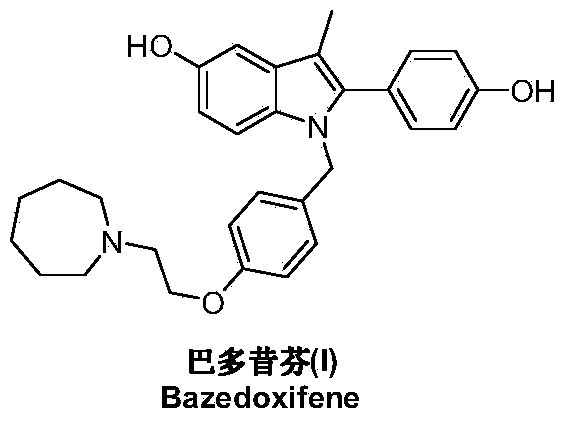
Preparation method of bazedoxifene
A technology of bazedoxifene and oxygen, which is applied in the field of preparation of a new generation of selective estrogen receptor modulator bazedoxifene, can solve the problems of difficult acquisition of raw materials, many reaction steps, and high pressure on environmental protection, and achieve product The yield and product purity are high, and the preparation process is fast and convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
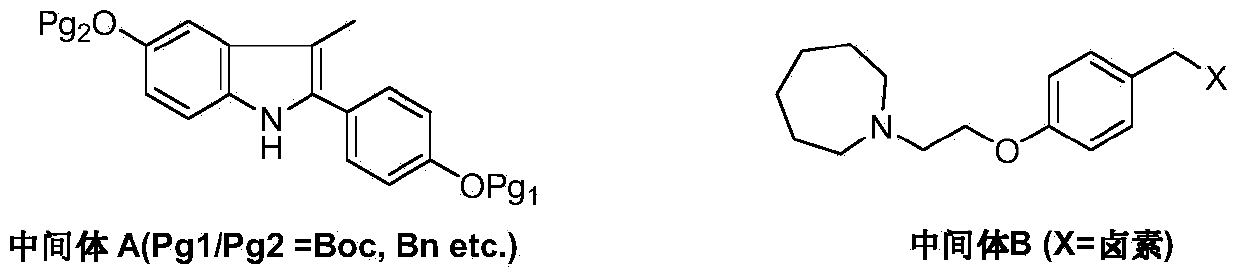
[0031] Under nitrogen protection, add 1-(4-methoxy-phenyl) propyne (II) (1.46g, 10mmol), N-[4-(2-azepan-1-yl) in the reaction flask -ethoxy-benzyl)]-N-[4-(methoxyphenyl)]hydrazine (III) (6.64g, 18mmol), bis(dimethylamino)bis(pentafluorobenzenethiol) ( Dimethylamino)titanium (76.5 mg, 0.2 mmol) and 50 mL of toluene were heated up to 100° C. and stirred for 36 hours. Anhydrous zinc chloride (4.1 g, 30 mmol) was added, the temperature was raised to reflux, and the reaction was continued for 24 hours, and TLC detected that the reaction was complete. The reaction solution was concentrated, dissolved in dichloromethane, washed twice with water, the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was recrystallized from ethanol to obtain off-white solid 1-[4-(2-azepan-1-yl-ethoxy-benzyl)]-2-(4-methoxy-phenyl)- 3-Methyl-5-methoxy-1H-indole (IV) 3.8g, yield 76.3%.
Embodiment 2
[0033] Under nitrogen protection, 1-(4-benzyloxy-phenyl) propyne (II) (2.22g, 10mmol), N-[4-(2-azepan-1-yl) -Ethoxy-benzyl)]-N-[4-(benzyloxyphenyl)]hydrazine (III) (9.0g, 20mmol), bis(dimethylamino)bis(pentafluorobenzenethiol) ( Dimethylamino)titanium (76.5 mg, 0.2 mmol) and 50 mL of toluene were heated up to 100° C. and stirred for 40 hours. Anhydrous zinc chloride (4.1g, 30mmol) was added, the temperature was raised to reflux, and the reaction was continued for 20 hours, and TLC detected that the reaction was complete. The reaction solution was concentrated, dissolved in dichloromethane, washed twice with water, the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was recrystallized from ethanol to obtain off-white solid 1-[4-(2-azepan-1-yl-ethoxy-benzyl)]-2-(4-benzyloxy-phenyl)- 5.1 g of 3-methyl-5-benzyloxy-1H-indole (IV), yield 78.5%.
Embodiment 3
[0035] Under nitrogen protection and at -78°C, add 1-[4-(2-azepan-1-yl-ethoxy-benzyl)]-2-(4-methoxy-benzene base)-3-methyl-5-methoxy-1H-indole (IV) (2.5g, 5mmol) and dichloromethane 25mL. Under stirring, a solution of boron tribromide (2.5 g, 10 mmol) in 25 mL of dichloromethane was added dropwise, and the reaction was carried out at room temperature for 10 hours. The reaction was quenched by adding saturated ammonium chloride solution, and the organic layer was separated, dried and concentrated under reduced pressure. The residue was recrystallized from ethyl acetate and n-hexane (1 / 1) to obtain 2.0 g of off-white solid bazedoxifene (I), with a yield of 85.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com