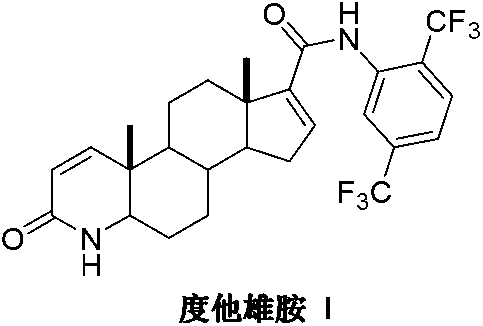
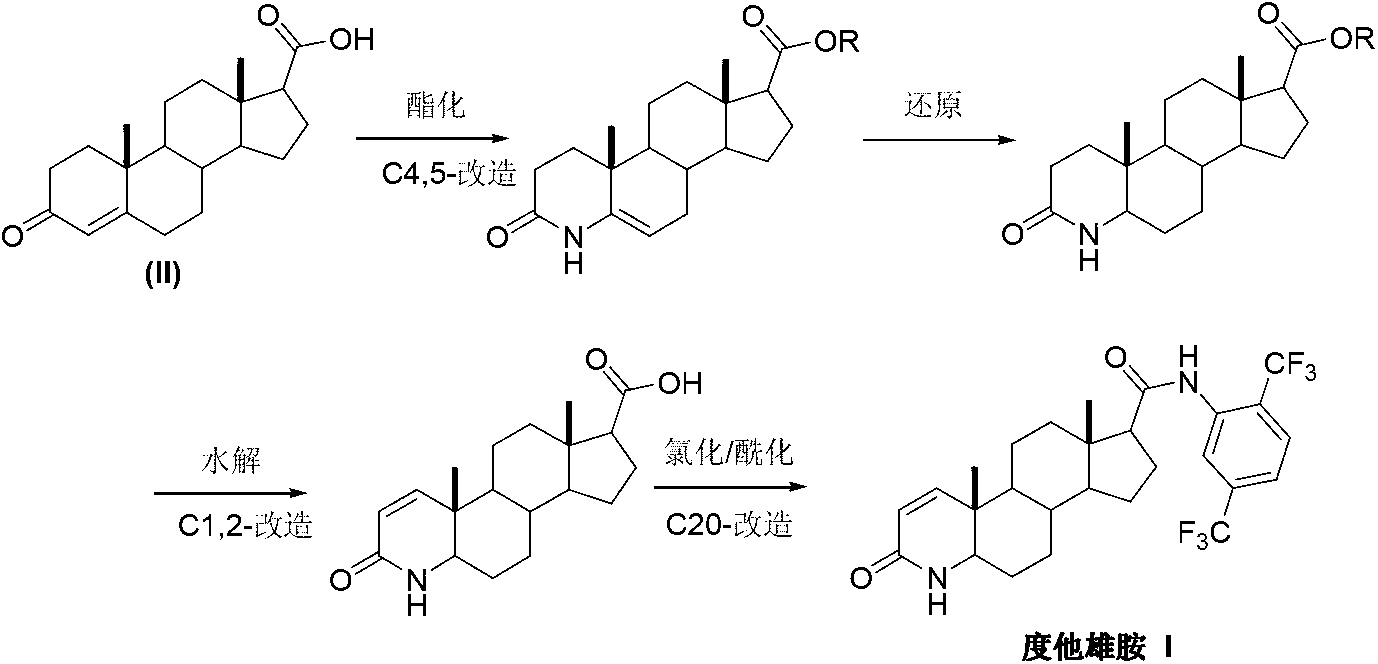
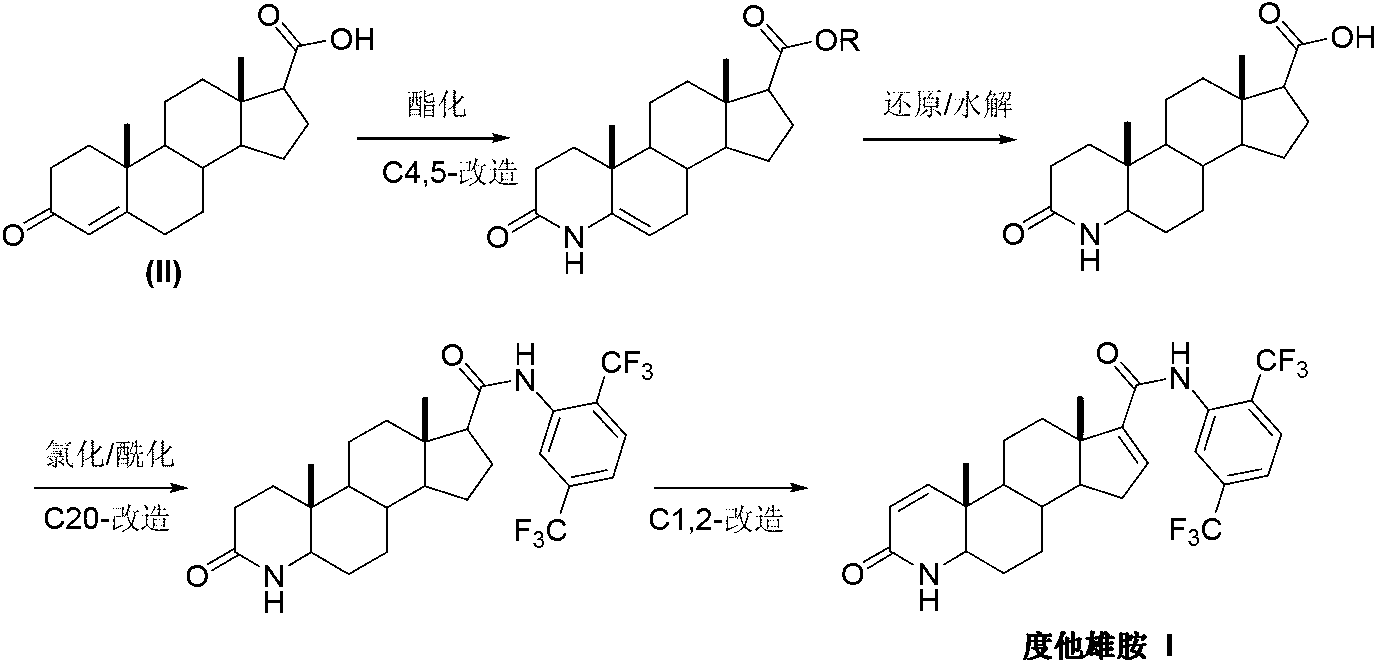
Preparation method of dutasteride
A technology of dutasteride and androsteride, applied in the field of preparation of dutasteride, can solve problems such as increased cost, decreased total yield, increased reaction steps, etc., and achieves the improvement of product quality, controllable production, and promotion of development. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add pregnenone acid (II) (3.16g, 10mmol) and 35mL of 20% ammonia water into the reaction flask, slowly raise the temperature to 75°C, and react until the system is uniformly dissolved. Continue to heat up to 85°C, and react until the system becomes cloudy again. Add 50 mL of xylene, raise the temperature to reflux, and separate water under reflux until the system becomes clear again. After finishing the reaction, it was concentrated under reduced pressure, and a solid precipitated out. After filtering, the filter cake was washed with toluene and dried in vacuum to obtain 3.10 g of white solid androst-4-en-3-one-17β-carboxamide (III), with a yield of 98.4%.
Embodiment 2
[0031] Add pregnenone acid (II) (3.16g, 10mmol), 50mL of xylene and 5mL of water into the reaction flask, slowly raise the temperature to 75°C, and at the same time pass ammonia gas, and react until the system is uniformly dissolved. Continue to heat up to 85°C, and react until the system becomes cloudy again. Continue to heat up to reflux, and divide water under reflux until the system becomes clear again. After finishing the reaction, it was concentrated under reduced pressure, and a solid precipitated out. After filtering, the filter cake was washed with toluene and dried in vacuo to obtain 3.08 g of white solid androst-4-en-3-one-17β-carboxamide (III), with a yield of 97.8%.
Embodiment 3
[0033] Add 4-aza-androst-5-en-3-one-17β-carboxamide (IV) (3.16g, 10mmol), 5% palladium carbon (0.16g, 5%w / w) into the hydrogenation reaction kettle , 1mL of acetic acid and 50mL of methanol, according to the hydrogenation reaction operating procedures, feed hydrogen. Add to keep the temperature at 20-35° C. and the pressure at 2-3 kg, continue the reaction for 10 hours, and the reaction ends. Concentrate under reduced pressure, and recrystallize the solid from acetone to obtain 3.02 g of 4-aza-5α-androst-3-one-17β-carboxamide (V), with a yield of 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com