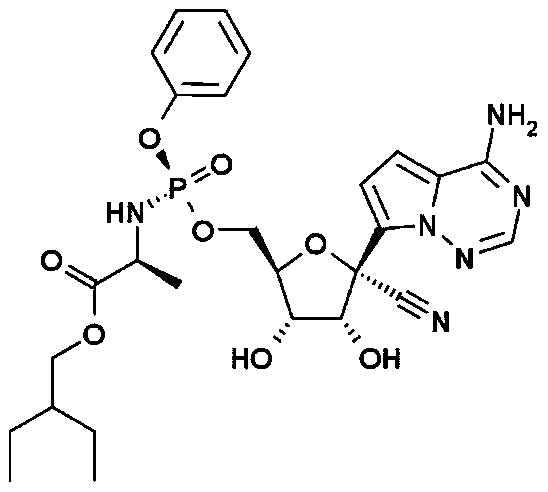
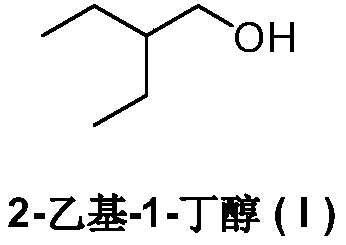
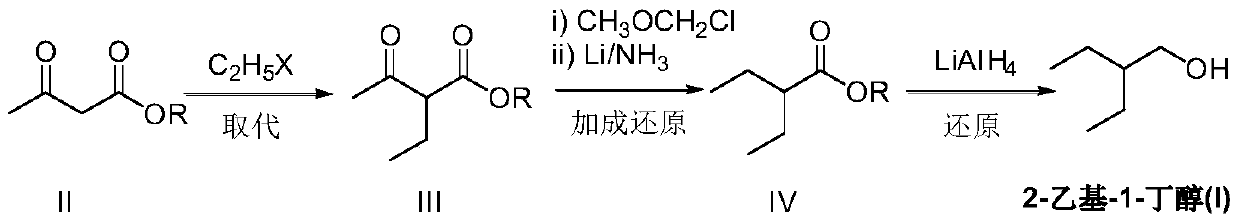
Preparation method of remdesivir intermediate 2-ethyl-1-butanol
A technology for remdesivir and intermediates, which is applied in the field of preparation of remdesivir intermediate remdesivir intermediate 2-ethyl-1-butanol, can solve the problem that there is no effective method for compounds and is not suitable for industrial production , the reaction conditions are difficult to control and other problems, to achieve the effect of convenient access, promotion of development, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Under a nitrogen atmosphere, add methyl acetoacetate (II) (11.6g, 100mmol) and 80mL of dry methanol to a dry reaction flask, cool down to -10°C, and slowly add 80mL of a methanol solution of sodium methoxide (8.1g, 150mmol) dropwise . After the dropwise addition was completed, ethyl iodide (18.7 g, 120 mmol) was added at -5-5°C and stirred for 2 hours. Raise the temperature to 5-10°C, and continue stirring for 3-4 hours. The reaction was quenched with 25 mL of saturated ammonium chloride, extracted three times with dichloromethane, the organic phases were combined, washed successively with 5% sodium bicarbonate solution and saturated brine, and dried over anhydrous magnesium sulfate. Concentration gave 13.4 g of light yellow liquid 2-ethyl-3-oxo-butyric acid methyl ester (III), yield 93.1%, EI-MS m / z: 145[M+H] + .
Embodiment 2
[0048] Under nitrogen atmosphere and at -5~0°C, add 2-ethyl-3-oxo-butyric acid methyl ester (III) (7.2g, 50mmol), sodium hydride (1.32g, 55mmol) and Solvent hexamethylphosphoric triamide (HMPA) 100mL. After rising to room temperature and stirring for 1 hour, chloromethyl methyl ether (4.4 g, 55 mmol) was added, and stirring was continued at room temperature for 2 to 3 hours. The reaction was quenched with saturated sodium bicarbonate solution under ice-cooling, and extracted three times with ether, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The resulting residue was transferred to a dry reaction flask, and 200 mL of diethyl ether was added to dissolve it. Under a nitrogen atmosphere, add a liquid ammonia solution containing lithium (0.35g, 50mmol), keep the reaction temperature at -35~-25°C and stir the reaction for 1 hour, slowly rise to room temperature, quench the reaction with saturated ammoni...
Embodiment 3
[0050] In a dry three-necked reaction flask under a nitrogen atmosphere, lithium aluminum hydride (3.8 g, 100 mmol) and ether 100 mL were added. The temperature was lowered to -5-0°C, and a diethyl ether solution of methyl 2-ethylbutyrate (IV) (13.0 g, 100 mmol) was added dropwise with stirring, and the addition was completed in about 1 hour. Continue the reaction at 0-5°C for 2 hours, quench the reaction with saturated ammonium chloride aqueous solution, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. 2-Ethyl-1-butanol (I) 9.1g, yield 89.2%, EI-MS m / z: 103[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com