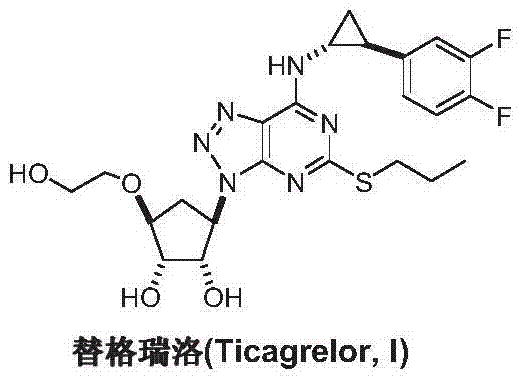
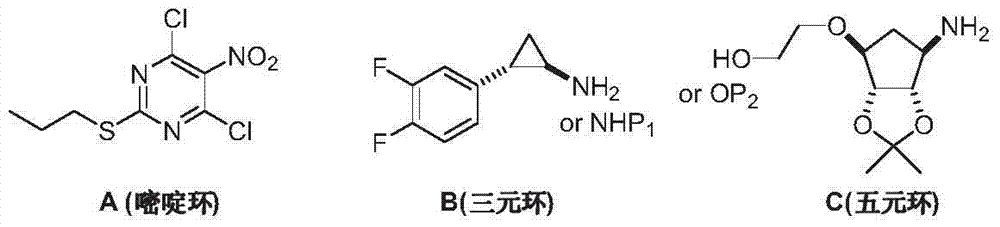
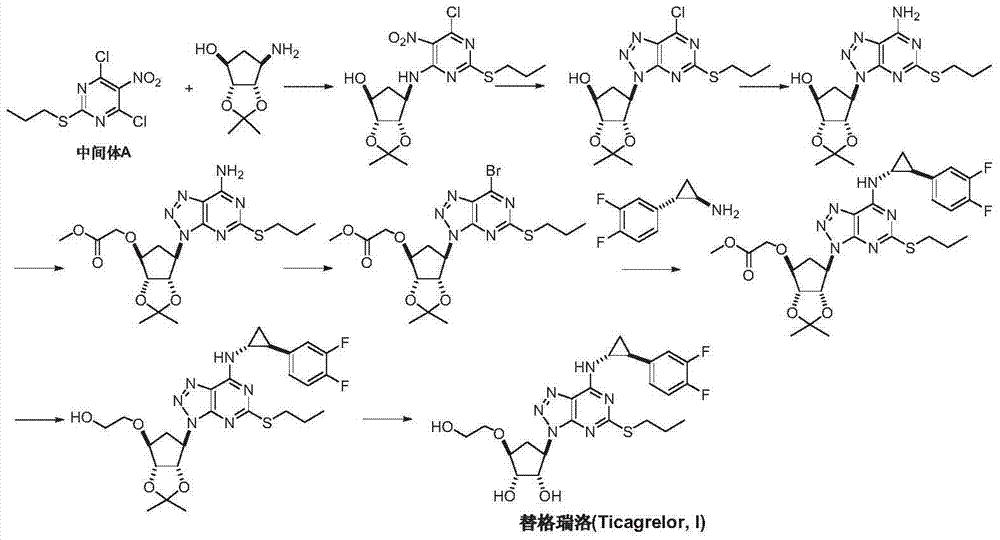
Preparation method of Ticagrelor intermediate 4, 6-dichloro-5-nitro-2-(propylthio) pyrimidine
A technology of ticagrelor and an intermediate, which is applied in the field of preparation of ticagrelor intermediate 4,6-dichloro-5-nitro-2-pyrimidine, can solve the problems of unfavorable industrial scale, equipment, environmental protection and safety Disadvantages and other problems, to achieve the effect of simple preparation process and promoting economic and technological development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 2-nitro-1,3-diethyl malonate (I) (10.3g, 50mmol), thiourea (4.76g, 62.5mmol, 1.25eq) and 100mL of methanol into a dry reaction flask, start stirring and Warm to reflux. 12 g of 30% methanol solution of sodium methoxide was added dropwise, and the reaction was kept under reflux for about 5 hours. TLC detected that the reaction was complete. Cool to room temperature, filter, wash the filter cake with cold methanol, and dry under vacuum at 50-55°C to obtain 9.5 g of light yellow solid 5-acetylamino-2-thio-barbiturate sodium (III), yield 90.0% .
Embodiment 2
[0028] Add 5-nitro-2-thio-barbiturate sodium (III) (8.5g, 40mmol) into 50mL water and 50mL methanol solution in a reaction flask, add bromo-n-propane (IV) dropwise at room temperature (5.37g, 44mmol, 1.1eq), after stirring for 15 minutes, slowly add 20mL of 10% sodium hydroxide solution dropwise and keep at room temperature, continue stirring for about 20 hours, and TLC detects that the reaction is complete. Add 100 mL of water, adjust the pH to 1.8-2.2 with dilute hydrochloric acid, extract three times with toluene, combine the organic phases, wash with saline and water, dry over anhydrous sodium sulfate, and recover toluene under reduced pressure to obtain light brown oil 4,6-dihydroxy -5-nitro-2-(propylmercapto)pyrimidine (V) 7.5g, yield 81.2%.
Embodiment 3
[0030] Add 4,6-dihydroxy-5-nitro-2-(propylthio)pyrimidine (V) (6.9g, 30mmol) and phosphorus oxychloride (15g) into the reaction flask, add N,N - Diisopropylethylamine (7.2 g), keeping the temperature not exceeding 25°C. After the addition, the temperature was raised to 110-115° C., and the reaction was carried out for 4 hours. TLC detected that the reaction was complete. Cool to room temperature, slowly pour into 100 mL of water, stir for 15 minutes, extract twice with toluene, combine organic phases, wash with saturated sodium bicarbonate, saturated brine and water successively, and dry over anhydrous sodium sulfate. After distillation under reduced pressure, 7.0 g of ticagrelor intermediate 4,6-dichloro-5-nitro-2-(propylthio)pyrimidine (intermediate A) was obtained as a pale yellow oil, with a yield of 87.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com