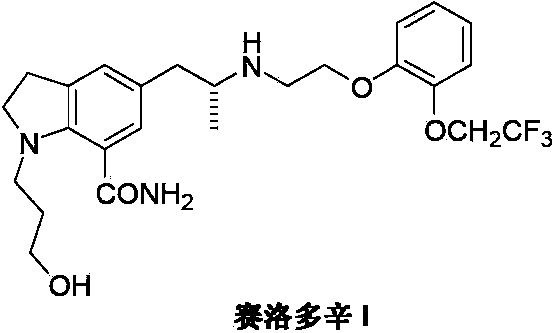
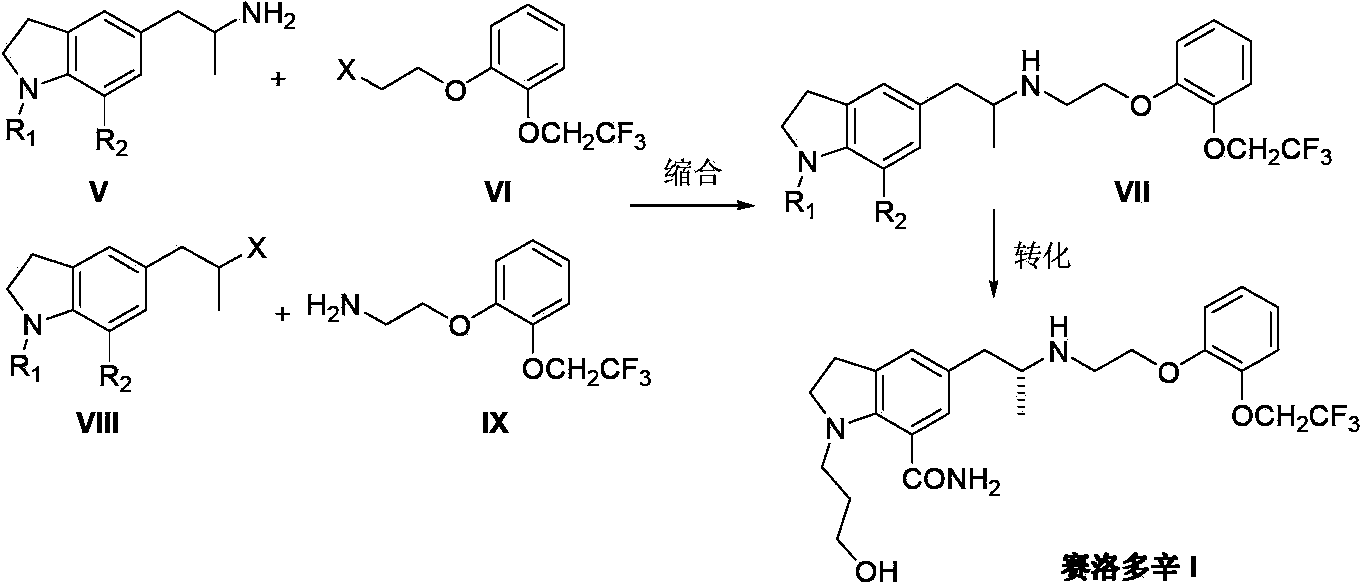
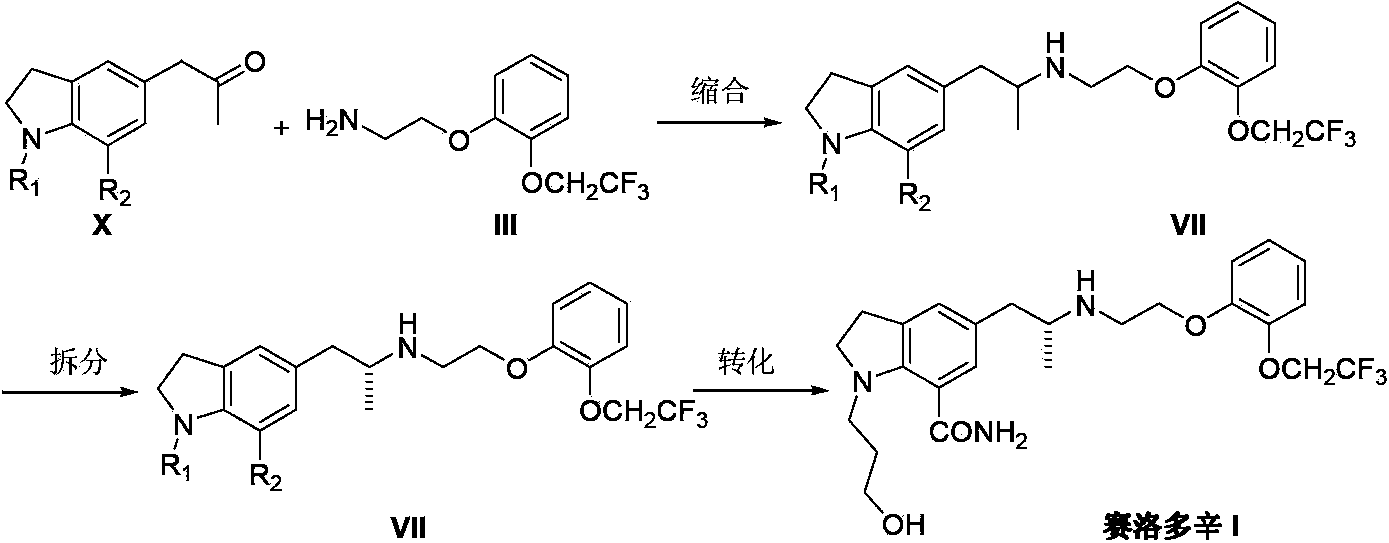
Preparation method of Silodosin
A technology of silodosin and disubstitution, which is applied in the field of preparation of silodosin, can solve problems affecting the industrialization process, poor selectivity, and difficult sources, and achieve the effects of promoting economic and technological development, controlling costs, and simple processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 1-(3-hydroxypropyl)-7-cyano-5-(2-oxopropyl)indoline (II) (2.58g, 10mmol), 2-[2- (2,2,2-Trifluoroethoxy)phenoxy]ethylamine (III) (2.58g, 11mmol) and 1,2-dichloroethane 35mL, add triacetoxyboron under nitrogen atmosphere at room temperature Sodium hydride (3.0g, 14mmol) and acetic acid (0.9g, 15mmol) were heated to 45-50°C and stirred for 20 hours. TLC detected that the reaction was complete. The reaction was quenched by adding 1N sodium hydroxide, extracted twice with 1,2-dichloroethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 2,3-dihydro-1-(3- Hydroxypropyl)-7-cyano-5-[2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]-1H-indole (IV) 4.1 g, yield 86.0%.
Embodiment 2
[0031] Add 1-(3-hydroxypropyl)-7-carboxamido-5-(2-oxopropyl)indoline(II) (2.76g, 10mmol), 2-[2 -(2,2,2-Trifluoroethoxy)phenoxy]ethylamine (III) (2.58g, 11mmol) and 1,2-dichloroethane 50mL, add triacetoxy group under nitrogen atmosphere at room temperature Sodium borohydride (3.0 g, 14 mmol) and acetic acid (0.9 g, 15 mmol) were heated to 50-55° C., stirred for 24 hours, and TLC detected the completion of the reaction. The reaction was quenched by adding 1N sodium hydroxide, extracted twice with 1,2-dichloroethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 2,3-dihydro-1-(3- Hydroxypropyl)-7-carboxamido-5-[2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]-1H-indyl Dole (IV) 4.0g, yield 80.8%.
Embodiment 3
[0033] Add 2,3-dihydro-1-(3-hydroxypropyl)-7-carboxamido-5-[2-[2-[2-(2,2,2-trifluoroethoxy) into the reaction flask Yl)phenoxy]ethylamino]propyl]-1H-indole(IV) (2.5g, 5mmol) and methanol 40mL, add S-(+)-mandelic acid (0.8g, 5mmol) under stirring, and Place in an ultrasonic generator and shake for 30 minutes, filter the insoluble matter, wash the filter cake with 10 mL of a mixture of ether and methanol, and dissolve the dried white solid with 40 mL of ethyl acetate and 40 mL of 10% sodium hydroxide, and stir at room temperature for 1 hour. After standing for liquid separation, the organic phase was dried with anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 0.88 g of white solid xerodoxine (I) with a yield of 35.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com