Preparation method for drug apixaban intermediate preventing venous thrombosis after hip and knee replacement surgeries
A technology of venous thrombosis and apixaban, which is applied in the field of medicine, can solve the problems of long route, low yield, and difficult purification, and achieve the effect of high product purity, high product yield, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
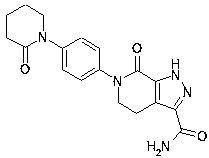
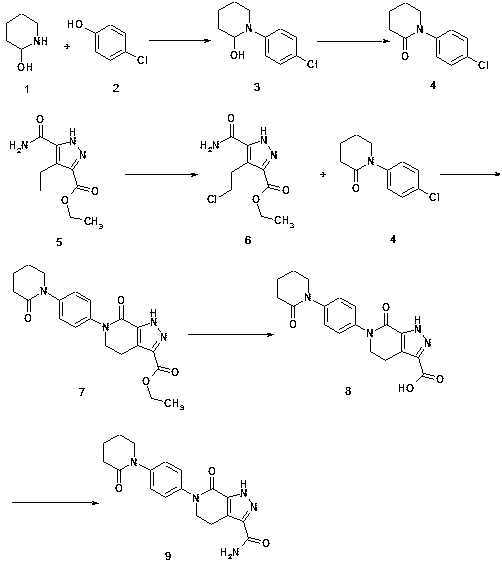
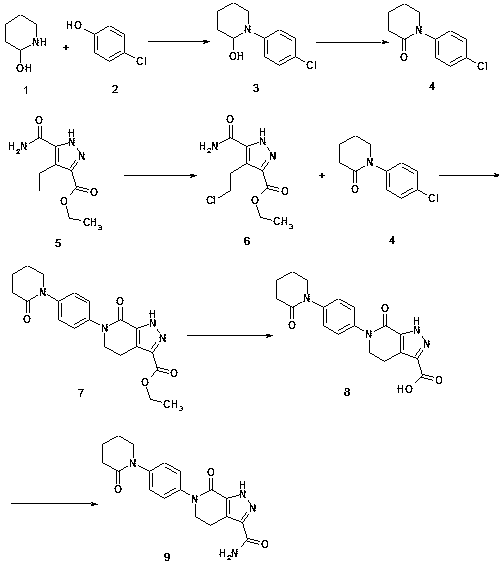
[0030] The preparation method of the drug apixaban intermediate for preventing venous thrombosis after hip and knee replacement surgery comprises the following process steps:
[0031] (1) Mix 2-hydroxypiperidine ① (5g, 1.6mol) with p-hydroxychlorobenzene ② (7g, 1.8mol), and add catalyst bisulfite in the presence of 85% concentrated sulfuric acid solution (30ml) of inorganic acid solution Sodium was thoroughly mixed and stirred, and an alkylation reaction occurred at 2.7MPa and 230°C to generate (2-hydroxypiperidin-1-yl)chlorobenzene③ (9.6g, 2.92mol);
[0032] (2) (2-hydroxypiperidin-1-yl) chlorobenzene ③ (9.6g, 2.92mol) generated in step (1) was added as catalyst ferric chloride, and dilute sulfuric acid (30ml) and water (40ml) Fully mixed and stirred in the presence of the mixed solution to generate (2-oxopiperidin-1-yl)chlorobenzene ④ (7.78g, 1.9mol);
[0033] (3) Dissolve ethyl 5-formamido-4-ethyl-pyrazole-3-carboxylate ⑤ (8g, 2.2mol) in the organic solvent carbon tetrachl...
Embodiment 2
[0040] The preparation method of the drug apixaban intermediate for preventing venous thrombosis after hip and knee replacement surgery comprises the following process steps:
[0041] (1) Mix 2-hydroxypiperidine ① (16.5g, 2.0mol) with p-hydroxychlorobenzene ② (15g, 1.8mol), and add catalyst sulfurous acid in the presence of 85% concentrated sulfuric acid solution (80ml) of inorganic acid Sodium hydrogen was thoroughly mixed and stirred, and an alkylation reaction occurred at 2.7MPa and 230°C to generate (2-hydroxypiperidin-1-yl)chlorobenzene③ (25.2g, 3.9mol);
[0042] (2) (2-hydroxypiperidin-1-yl) chlorobenzene ③ (25.2g, 3.9mol) generated in step (1) is added with catalyst iron trichloride, and dilute sulfuric acid (70ml) and water (100ml) Fully mixed and stirred in the presence of the mixed solution to generate (2-oxopiperidin-1-yl)chlorobenzene ④ (20.16g, 3.7mol);
[0043](3) Dissolve ethyl 5-formamido-4-ethyl-pyrazole-3-carboxylate ⑤ (20g, 2.57mol) in the organic solvent c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com