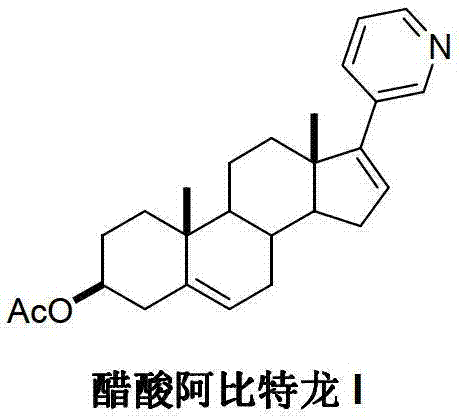
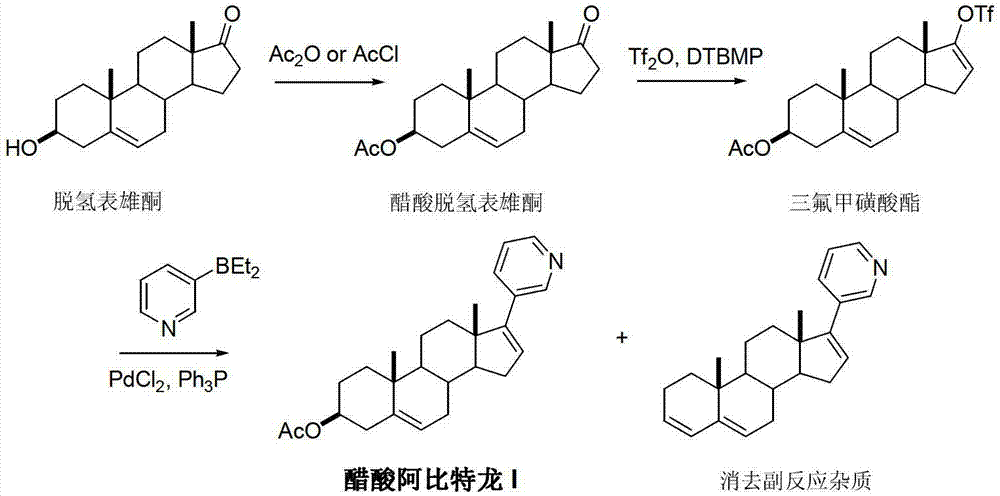
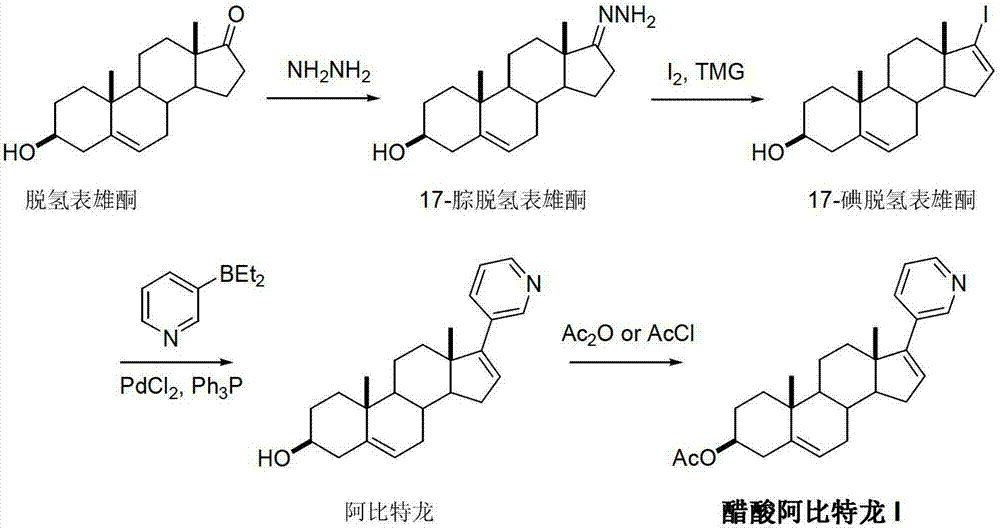
Preparation method of abiraterone acetate
A technology of abiraterone acetate and alcohol acetate, which is applied in the field of preparation of abiraterone acetate, can solve the problems of production cost, reaction yield and insufficient discharge of three wastes, and achieve the promotion of economic and technological development, easy post-processing and purification, The effect of high chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 17-halogen-androst-5,16-dien-3β-alcohol acetate (II) (4.4g, 10mmol), 3-pyridineboronic acid pinacacate (III) (2.25g, 11mmol) into the reaction flask , Potassium acetate (1.2g, 12mmol), 50mL of dimethyl sulfoxide, heated up to 40°C, and stirred until the system was dissolved uniformly. Under the protection of nitrogen, 1,1'-bis(diphenylphosphino)ferrocene nickel chloride (0.18g, 2.5%eq) was added, the temperature was raised to 80°C, and the reaction was continued for 5 hours, and the reaction was detected by TLC. The solvent was recovered under reduced pressure. The residue was dissolved in dichloromethane and water, and the aqueous layer was separated, washed successively with 10% sodium carbonate solution and saturated brine, and dried over anhydrous magnesium sulfate. Dichloromethane was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate and n-hexane to obtain 3.64 g of abiraterone acetate (I), with a yield of 93.1%.
Embodiment 2
[0036]Add 17-halogen-androst-5,16-dien-3β-alcohol acetate (II) (4.4g, 10mmol), 3-pyridineboronic acid pinacacate (III) (2.25g, 11mmol) into the reaction flask , potassium carbonate (1.66g, 12mmol), N,N-dimethylformamide 50mL and water 10mL, warm up to 40°C, and stir until the system is uniformly dissolved. Under nitrogen protection, bis(triphenylphosphine)palladium chloride (0.19 g, 2.5% eq) was added, the temperature was raised to 80° C., and the reaction was continued for 8 hours, and the reaction was detected by TLC. The solvent was recovered under reduced pressure. The residue was dissolved in dichloromethane and water, and the aqueous layer was separated, washed successively with 10% sodium carbonate solution and saturated brine, and dried over anhydrous magnesium sulfate. Dichloromethane was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate and n-hexane to obtain 3.33 g of abiraterone acetate (I), with a yield of 85.2%.
Embodiment 3
[0038] Add 17-halogen-androst-5,16-dien-3β-alcohol acetate (II) (4.4g, 10mmol), 3-pyridineboronic acid pinacacate (III) (2.25g, 11mmol) into the reaction flask , potassium phosphate (2.5g, 12mmol), 50mL of dioxane and 10mL of water, heated up to 40°C, and stirred until the system was dissolved uniformly. Under the protection of nitrogen, tetrakis(triphenylphosphine)palladium (0.29 g, 2.5% eq) was added, the temperature was raised to 80° C., and the reaction was continued for 8 hours, and the reaction was detected by TLC. The solvent was recovered under reduced pressure. The residue was dissolved in dichloromethane and water, and the aqueous layer was separated, washed successively with 10% sodium carbonate solution and saturated brine, and dried over anhydrous magnesium sulfate. Dichloromethane was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate and n-hexane to obtain 3.25 g of abiraterone acetate (I), with a yield of 83.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com