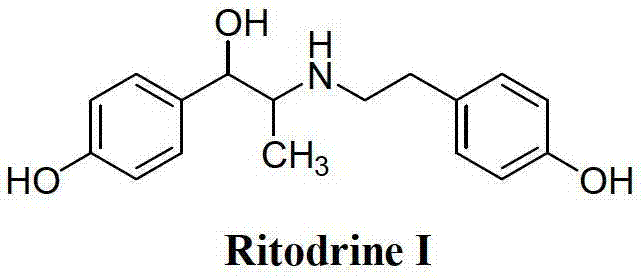
Preparation method of ritodrine
A technology of hydroxyphenyl and propanol hydrochloride, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of amino hydroxyl compounds, etc., to achieve high chemical selectivity, promote economic and technological development, and improve quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
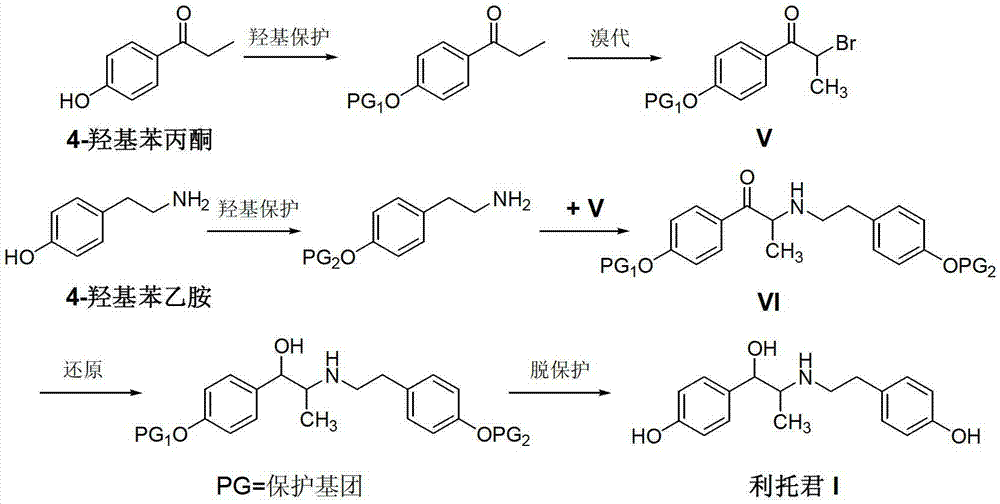
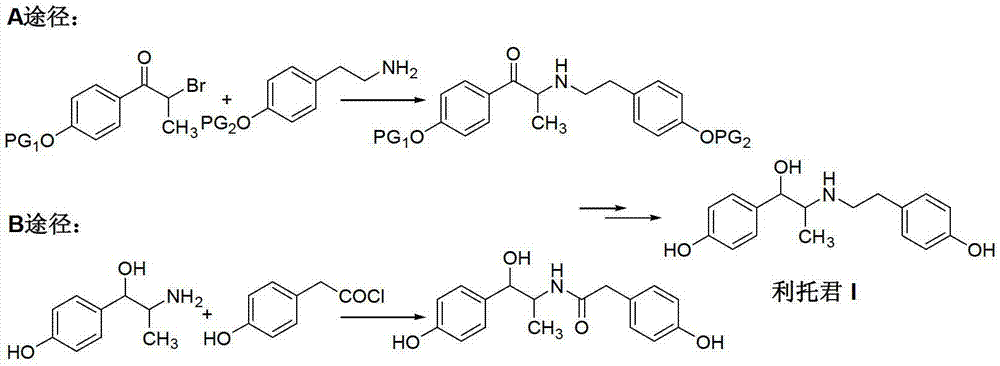
[0027] Add 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (II) (50.2g, 0.25mol), triethylamine (25.0g, 0.25mol) and dichloromethane into a 500mL three-necked flask 250mL, warm to 40-45°C, stir until the system is uniformly dissolved. When the temperature drops below 10°C, slowly add 4-hydroxyphenylacetyl chloride (III) (46.8g, 0.28mol) in dichloromethane solution dropwise for about 1 hour. The reaction was continued at room temperature for 6 hours, and TLC detected that the reaction was complete. The reaction solution was washed with 10% sodium bicarbonate solution and water, and dried with anhydrous sodium sulfate. The solvent was recovered under reduced pressure, and the residue was recrystallized from a mixed solvent of n-hexane and ethyl acetate to obtain an off-white solid N-(2-(4-alkoxyphenyl)-2-hydroxy-1-methylethyl)- 4-hydroxyphenylacetamide (IV) was 68.5 g, and the yield was 91.0%.
Embodiment 2
[0029] Add Intermediate (IV) (60.2g, 0.2mol), 5% palladium-carbon catalyst (6g, 10%w / w), 2mL of concentrated hydrochloric acid and 500mL of methanol into a 1L hydrogenation kettle, and maintain the hydrogen pressure according to the hydrogenation procedure 5KG and temperature 50°C, until no more hydrogen is absorbed. The temperature was lowered, the catalyst was recovered by filtration, and concentrated under reduced pressure. The residue was recrystallized with ethyl acetate to obtain 47.2 g of white solid ritodrine (I) with a yield of 82.2%.
Embodiment 3
[0031] Intermediate (IV) (60.2 g, 0.2 mol) and 250 mL of tetrahydrofuran were added to a 500 mL three-necked flask, and stirred until the system was uniformly dissolved. The temperature was lowered to 0°C, and lithium aluminum tetrahydrogen (11.4 g, 0.3 mol) was added to the reaction solution in batches. After the addition was completed, the temperature was raised to reflux, and the reaction was continued for 4 hours, and the reaction was completed by TLC detection. Reduce to room temperature, add 30 mL of 10% sodium hydroxide solution, and filter to remove insoluble materials. Tetrahydrofuran was recovered from the filtrate under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 48.5 g of white solid ritodrine (I) with a yield of 84.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com