Preparation method of Olaparib intermediate
An intermediate and oxo generation technology, which is applied in the field of preparation of olaparib intermediates, can solve problems such as unfavorable large-scale production control, poor reaction monitoring, etc., and achieve the effects of promoting development, easy access to raw materials, and environmentally friendly and economical processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
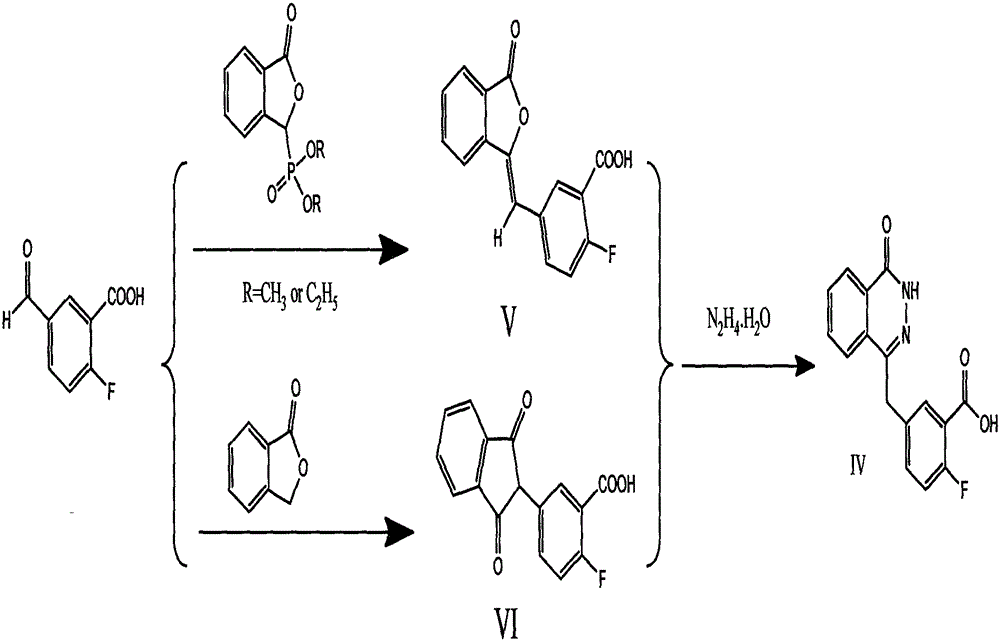
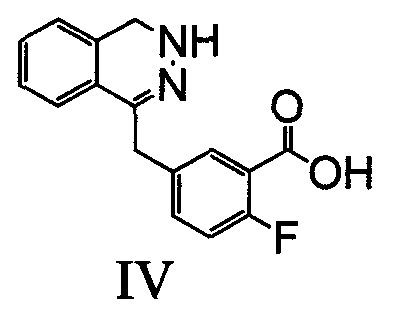
[0032] Example 1: Preparation of olaparib intermediate (IV): 2-fluoro-5-[(4-oxo-3,4-dihydrophenazin-1-yl)methyl]benzoic acid
[0033] 1.68 g of 2-fluoro-5-formylbenzoic acid and 2.5 g of dimethyl (3-oxo-1,3-dihydro-isobenzofuran-1-yl)phosphate were dissolved in 20 mL of dioxane, Add 1.4mL triethylamine dropwise at 0°C, continue the reaction until the raw materials are completely reacted, distill off the solvent under reduced pressure, add water and stir for 1 hour, filter with suction, and dry to obtain 2.0g of solid, namely intermediate (V): 2-Fluoro-5-(3-oxo-3H-isobenzofuran-1-ylmethylene)benzoic acid, yield; 70.4%. ESI-MS, m / z: 307.1 [M+Na] + , 285.1[M+H] + .
[0034] Dissolve 2.8g of intermediate V: 2-fluoro-5-(3-oxo-3H-isobenzofuran-1-ylmethylene)benzoic acid in 20mL of ethanol, add 8mL of hydrazine hydrate at room temperature, and reflux the reaction , until the raw material reaction is complete, concentrated under reduced pressure to remove the solvent, added water,...
Embodiment 2
[0035] Example 2: Preparation of olaparib intermediate (IV): 2-fluoro-5-[(4-oxo-3,4-dihydrophenazin-1-yl)methyl]benzoic acid
[0036]Dissolve 0.9g of 2-fluoro-5-formylbenzoic acid and 0.7g of phthalide in 100mL of ethyl propionate, add dropwise a solution of 1.3g of sodium methoxide in 20mL of methanol at room temperature, and keep the temperature constant with an ice-water bath during the dropwise addition. higher than 30°C. After dripping, raise the temperature and reflux, react until the raw materials are completely reacted, remove the solvent by distillation under reduced pressure, add water and stir for 1 hour, filter with suction, wash the filter cake with ethyl acetate, acidify the acetic acid solution, stir, wash with water after filtration, and dry to obtain 0.9g of solid , Intermediate (VI): 5-(2,3-dihydro-1,3-dioxo-1H-inden-2-yl)-2-fluorobenzoic acid, yield; 64.2%.
[0037] Dissolve 0.8g of intermediate (VI): 5-(2,3-dihydro-1,3-dioxo-1H-inden-2-yl)-2-fluorobenzoic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com