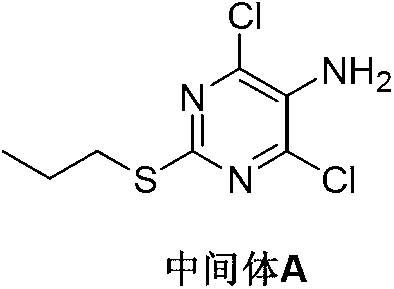
Preparation method of Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine
A technology of ticagrelor and aminopyrimidine is applied in the field of preparation of ticagrelor intermediate 4,6-dichloro-2--5-aminopyrimidine, and can solve the problem of difficult filtration, large amount of solvent for extraction, and amount of solid waste. major problems, to achieve the effect of promoting economic technology, simplifying nitro reduction, and promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Into a 1L reaction flask was added 4,6-dichloro-5-nitro-2-(propylmercapto)pyrimidine (V) (26.6g, 0.1mol), sodium hydrosulfite (43.5g, 0.25mol), methanol 250mL and Water 250mL, start stirring, keep stirring and reacting at 55 DEG C for 5 hours, TLC detects that the reaction is complete. Concentrate to half the volume under reduced pressure and extract three times with dichloromethane. The organic phases were combined, washed with 5% sodium bicarbonate and water successively, dried, and the solvent was recovered by distillation under reduced pressure.
Embodiment 2
[0033] Into a 1L reaction flask, add 4,6-dichloro-5-nitro-2-(propylmercapto)pyrimidine (V) (26.6g, 0.1mol), sodium hydrosulfite (69.6g, 0.40mol), 250mL of acetonitrile and Water 250mL, start stirring, keep stirring and reacting at 55 DEG C for 4 hours, TLC detects that the reaction is complete. Concentrate to half the volume under reduced pressure and extract three times with toluene. The organic phases were combined, washed successively with 5% sodium bicarbonate and water, dried, and the solvent was recovered by distillation under reduced pressure. The residual oil was frozen and crystallized with isopropyl ether to obtain a pale yellow solid Intermediate A21.7g with a yield of 91.6%.
Embodiment 3
[0035] Into a 1L reaction flask, add 4,6-dichloro-5-nitro-2-(propylmercapto)pyrimidine (V) (26.6g, 0.1mol), sodium hydrosulfite (69.6g, 0.40mol), 250mL of toluene and 250 mL of water was added simultaneously with 2.5 g of tetrabutylammonium bromide. Start stirring, keep stirring and react at 65 DEG C for 4 hours, and TLC detects that the reaction is complete. Cooled to room temperature, the organic layer was separated, the aqueous layer was washed twice with toluene, the organic phases were combined, washed with 5% sodium bicarbonate and water in turn, dried, and distilled under reduced pressure to recover the solvent, and the residual oil was washed with methyl tert-butyl The ether was frozen and crystallized to obtain a light yellow solid intermediate A22.3g, the yield was 94.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com