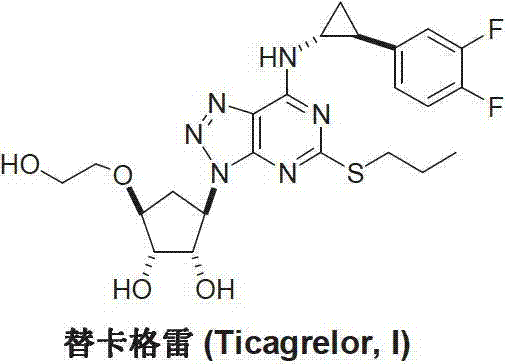
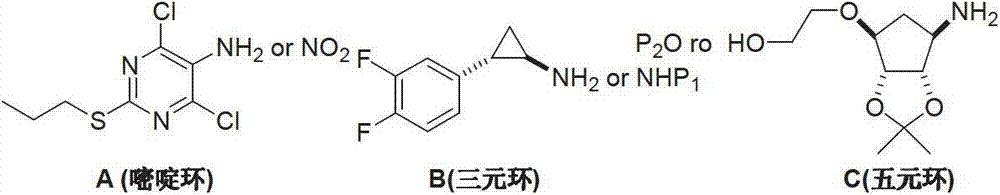
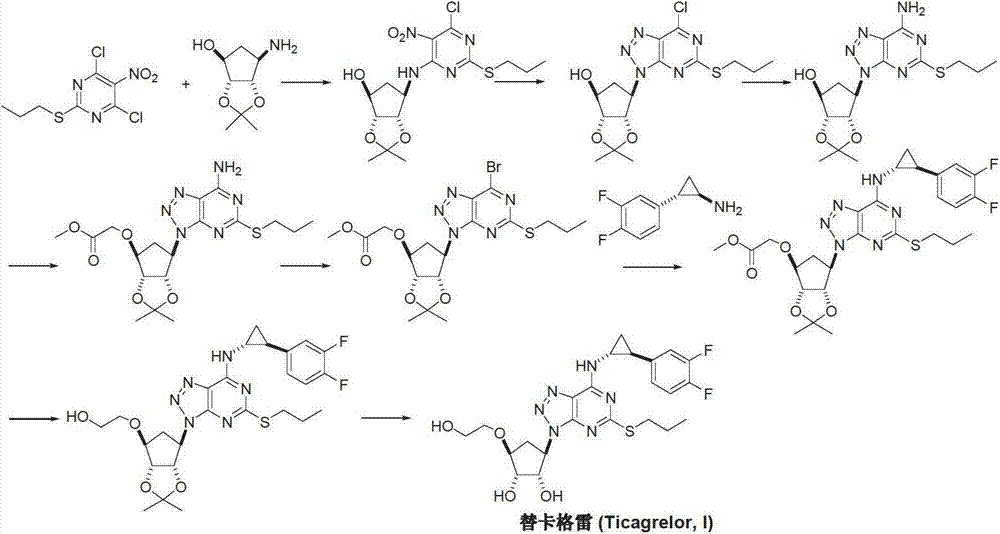
Preparation method of ticagrelor
A technology of ticagrelor and amino group is applied in the field of preparation of new anticoagulant drug ticagrelor, which can solve the problems of difficult control of coupling position, and achieve the effects of quick and convenient preparation, high product yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Under dry and nitrogen atmosphere, add 1-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3 -Dioxolane-4-oxyl]ethanol]-6-yl]-5-amino-4-carboxamido-1,2,3-triazole (II) (3.27g, 10mmol), ethanol Sodium (2.72g, 40mmol) and absolute ethanol 100mL were slowly heated to reflux and reacted for 3 hours. Dimethyl carbonate (2.7 g, 30 mmol) was added dropwise over half an hour and reflux was continued for 6 hours. Ethanol was distilled off at atmospheric pressure, and the distillation was repeated once with fresh ethanol. Cool down to room temperature, add 50 mL of water, adjust the pH to 6 with dilute acid while stirring, slowly crystallize for 2 hours, filter, and recrystallize the filter cake with 50% methanol to obtain off-white solid 9-[3aR-(3aα, 4α, 6α ,6aα)-[[2,2-Dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxol-4-oxyl]ethanol]-6-yl]-2, 2.55 g of 6-dihydroxy-8-azapurine (IV), yield 72.5%.
Embodiment 2
[0044] Add 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxolane to the reaction flask -4-oxyl]ethanol]-6-yl]-2,6-dihydroxy-8-azapurine (IV) (1.77g, 5mmol), phosphorus oxychloride 15mL, start stirring, and cool down to 0°C , 3.5 mL of 2,6-lutidine was added dropwise. The temperature was slowly raised to 100°C, and the reaction was maintained at this temperature with stirring for 9 hours. Phosphorus oxychloride was recovered under reduced pressure, and the residue was cooled to room temperature, and the reaction was quenched with ice water. Extracted 3 times with dichloromethane, combined the organic phases, dried over anhydrous sodium sulfate, and recovered the solvent under reduced pressure to obtain the oil 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl -Tetrahydro-4H-cyclopentadieno-1,3-dioxol-4-oxyl]ethanol]-6-yl]-2,6-dichloro-8-azapurine (V) 1.7 g, yield 87.5%.
Embodiment 3
[0046] Add 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxolane to the reaction flask -4-oxyl]ethanol]-6-yl]-2,6-dichloro-8-azapurine (V) (1.95g, 5mmol), trans-(1R,2S)-2-(3, 4-Difluorophenyl)cyclopropylamine (VI) (1.0g, 6mmol) and 25mL of acetonitrile were stirred at room temperature, and 1.5mL of triethylamine was added. Keep stirring at room temperature for 12 hours, and TLC detects that the reaction is complete. Concentrate under reduced pressure, add ethyl acetate and water to the residue, and adjust pH=4 with dilute acid. The organic phase was separated and the aqueous phase was extracted 3 times with ethyl acetate. The organic phases were combined, washed with pure water and brine successively, dried, and the solvent was recovered by distillation under reduced pressure to obtain the oil 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl- Tetrahydro-4H-cyclopentadiene-1,3-dioxol-4-oxyl]ethanol]-6-yl]-6-[[(1R,2S)-2-(3,4 -Difluorophenyl)cyclopropyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com