Eltrombopag inclusion compound and preparations and preparation method thereof
A technology for eltrombopag and inclusion complex, which is applied in the field of eltrombopag inclusion complex and its preparation and preparation, can solve problems such as the need for further research, and achieves improving water solubility, avoiding curative effect reduction, and reducing chelation effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] Preparation of clathrates: including but not limited to freeze-drying method: Weigh each component separately, dissolve the clathrate material completely in water, add the drug, stir, centrifuge, take the supernatant, and then freeze the supernatant Dry to obtain clathrate.
[0051] Preparation of capsules: including but not limited to powder method: specifically, weigh each composition separately, make inclusion compound of Eltrombopag according to the above-mentioned inclusion compound preparation method, sieve the inclusion compound, add disintegrant, Part of the filler, mixed, then add the rest of the filler, mix, finally add lubricant, mix evenly and fill in capsules (enteric-coated capsules or gastric-coated capsules), to obtain capsules for in vitro dissolution test.
[0052] Preparation of tablets: including but not limited to powder direct compression method: specifically, each component is weighed separately, and Eltrombopag is prepared into an inclusion compo...
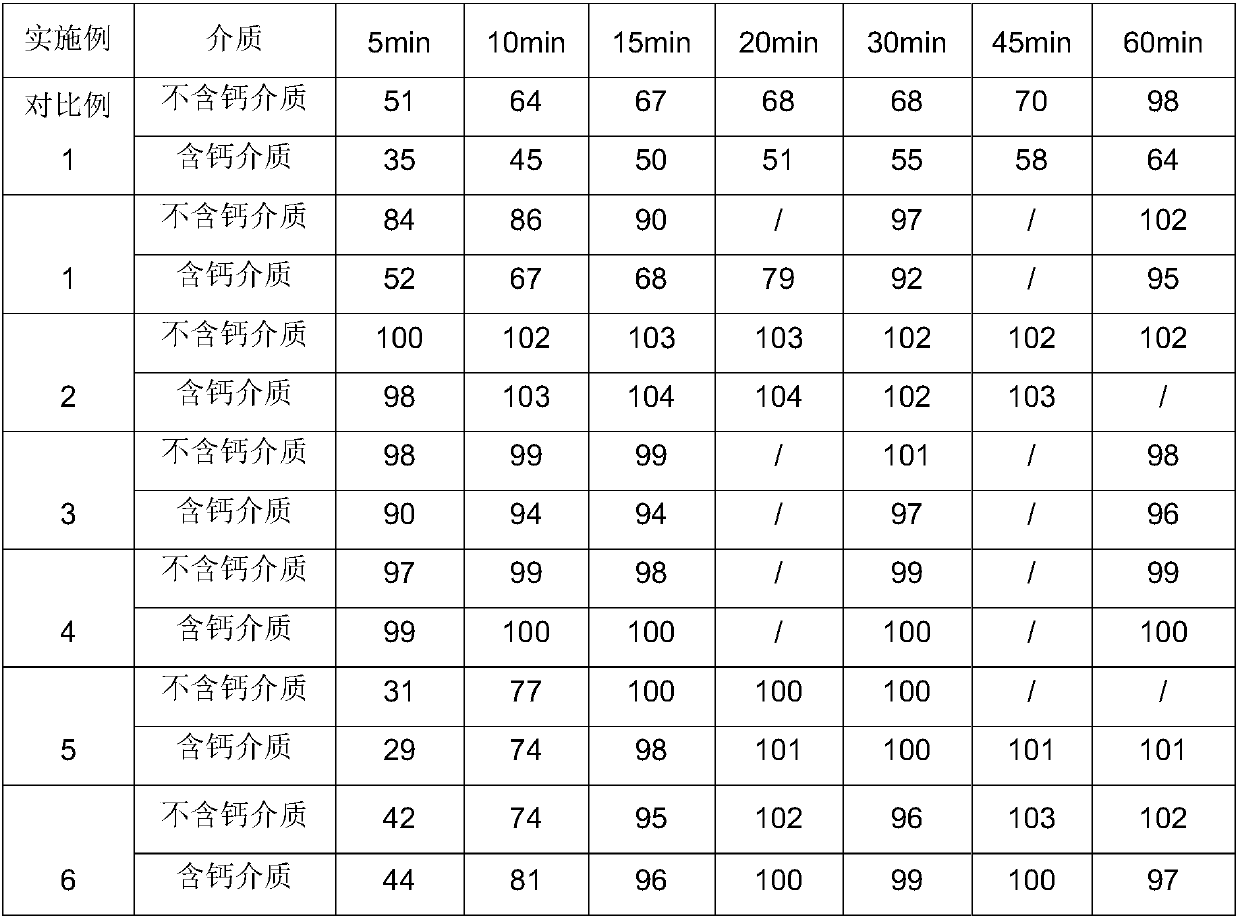
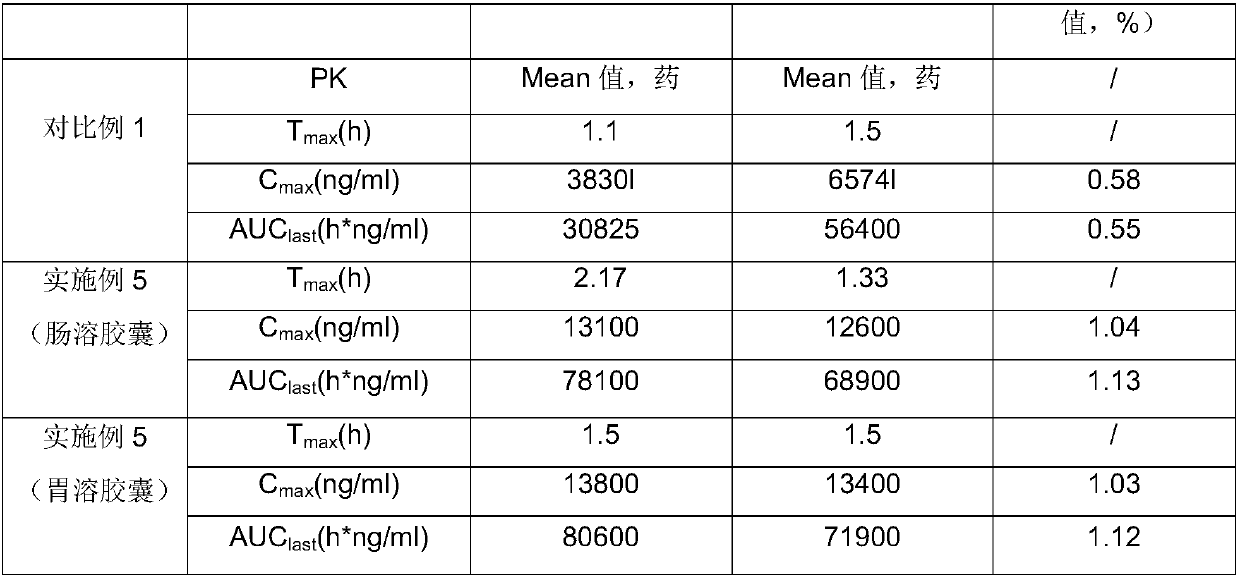
Embodiment 1
[0060] In this embodiment, according to the above-mentioned preparation process and test conditions, the Eltrombopag inclusion compound was dissolved in pH6.8 phosphate buffer solution containing 0.5% by weight SDS, containing 2.78mmol / L calcium chloride and containing 0.5% by weight After the pH 6.8 phosphate buffer solution medium solution of %SDS is filtered, the two filtrates are subjected to HPLC test respectively. Wherein, the concrete composition of clathrate is as follows:
[0061] prescription composition
Prescription ratio (weight%)
Eltrombopag olamine
1.43
8.96
89.61
total
100.00
[0062] And in this example, the clathrate was prepared by freeze-drying method, weighed separately, the weight ratio of Eltrombopag to α-cyclodextrin was 1:6.3, dissolved α-cyclodextrin in purified water, and waited After the solution is clarified, dissolve Eltrombopag in α-cyclodextrin aqueous soluti...
Embodiment 2
[0065] In this example, according to the preparation process and test conditions substantially the same as in Example 1, the Eltrombopag inclusion complex was dissolved in a pH6.8 phosphate buffer solution containing 0.5% by weight of SDS, containing 2.78mmol / L chlorine After the solution of calcium chloride in a pH 6.8 phosphate buffer solution medium containing 0.5% by weight SDS was filtered, HPLC tests were performed on the two filtrates respectively. The difference is that, in this embodiment, the specific composition of Eltrombopag inclusion compound is as follows:
[0066] prescription composition
Prescription ratio (weight%)
Eltrombopag olamine
1.48
β-cyclodextrin
16.42
82.10
total
100.00
[0067] Moreover, the weight ratio of Eltrombopag to β-cyclodextrin is 1:11.1, after Eltrombopag is dissolved in β-cyclodextrin aqueous solution, stir in a water bath at 70°C. The inclusion rate of the Eltrombo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com