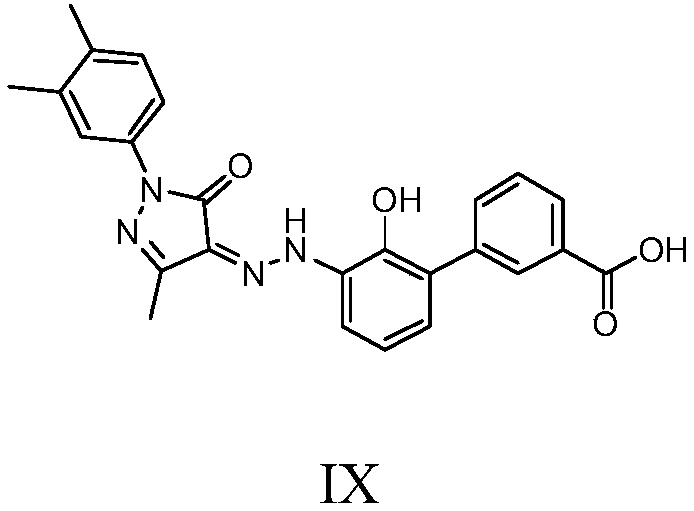
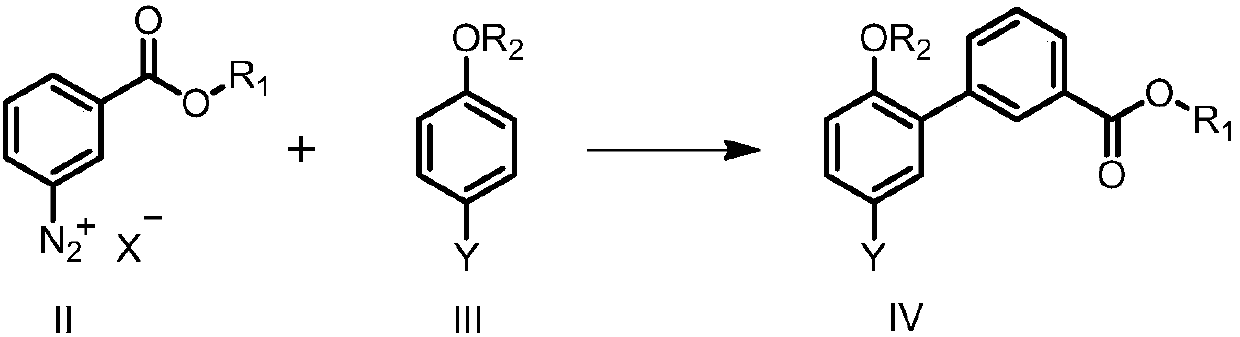
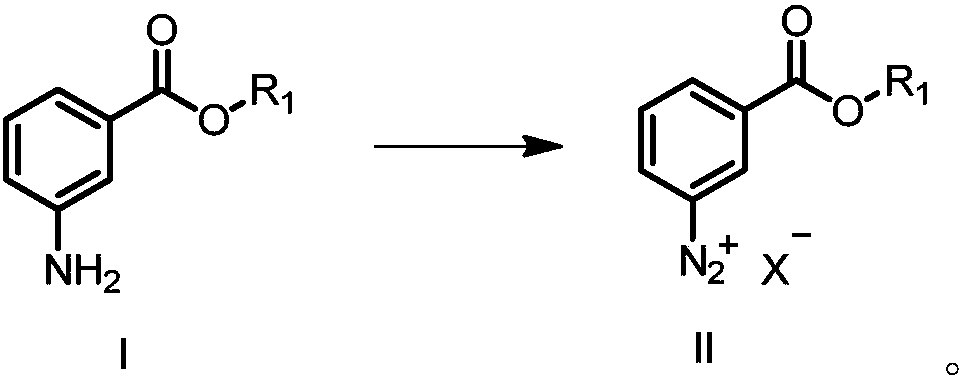
Preparation method of eltrombopag
A benzyl compound technology, applied in the field of preparation of Eltrombopag, can solve the problems of no practical industrialization value, high production cost, and many reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Embodiment 1: Preparation of tetrafluoroboric acid-3-formic acid methyl benzene diazo
[0130] 3-Aminobenzoic acid methyl ester hydrochloride (191.0mmol) was dissolved in 120mL of water, then concentrated hydrochloric acid (398.0mmol) was added, cooled and stirred at low temperature to about -8°C, and the temperature was controlled at about -8°C. Sodium nitrate aqueous solution (191.0mmol sodium nitrite, 24mL water), temperature control about -8°C, stirring reaction for about 2h, temperature control about -3°C, slowly add sodium tetrafluoroborate aqueous solution (3.0eq. sodium tetrafluoroborate, 600mL water ), about 20 minutes after the dropwise addition was completed, the temperature was controlled at -3°C and the reaction was stirred for about 30 minutes. Filtered, the filter cake was washed once with 100mL ice water to obtain a white solid, namely 44.0g of methyl tetrafluoroborate-3-carboxylate diazobenzene, collected The rate is 91.7%.
Embodiment 2
[0131] Embodiment 2: the preparation of chloro-3-formic acid methyl benzene diazo
[0132] Dissolve 3-aminobenzoic acid methyl ester hydrochloride (16.0mmol) in 15mL of methanol, add concentrated hydrochloric acid (32.0mmol), cool and stir at low temperature to about -8°C, and slowly add dropwise to the above solution under temperature control of about -8°C Sodium nitrite aqueous solution (16.0mmol sodium nitrite, 2.0mL water), temperature control at about -8°C, stirring reaction for about 2h, filtering to obtain the filtrate, which is methyl chloride-3-carboxylate diazobenzyl alcohol solution, which can be used directly react in the next step.
Embodiment 3
[0133] Embodiment 3: the preparation of hydrosulfate-3-formic acid methyl benzene diazo
[0134] To a solution of methyl 3-aminobenzoate (19.87 mmol) in concentrated sulfuric acid (10 mL) was slowly added dropwise 1.56M nitrosylsulfuric acid at 0-5°C, and the addition was completed in about one hour. After dropping, keep warm at 0-5°C until the reaction of the raw materials is complete, and then the hydrosulfate-3-formic acid methyl diazobenzene solution is obtained, which can be directly used in the next reaction step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com