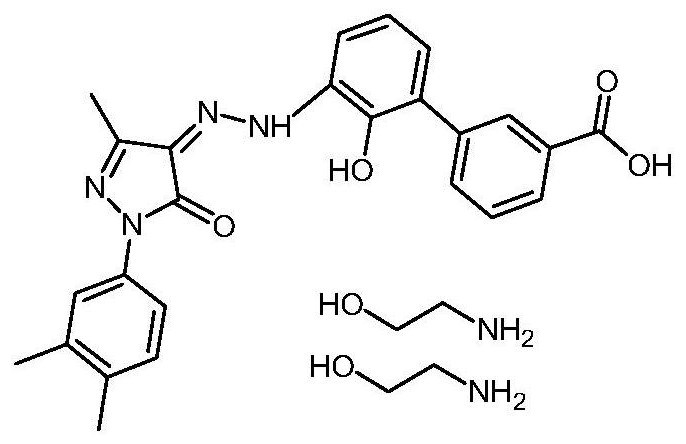
Eltrombopag diethanolamine salt and preparation method thereof
A technology of ethanolamine salt and ethanolamine, which is applied in the field of eltrombopag diethanolamine salt and its preparation, can solve the problems that solvent residues do not meet the ICH guidelines, the amount of tetrahydrofuran is too much, and it is difficult to industrialize production, so as to reduce the amount of solvent residues and equipment The effect of low requirements and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
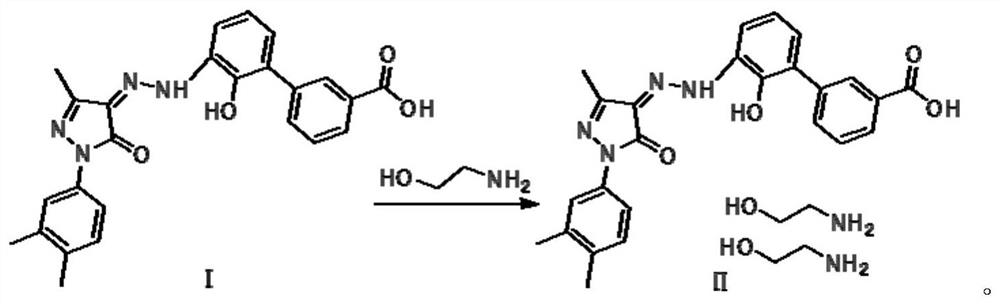
[0022] The embodiment of the present invention provides a preparation method of eltrombopag diethanolamine salt. The preparation method comprises the following steps: dissolving ethanolamine in an organic solvent, heating up, stirring and dissolving, adding the tetrahydrofuran solution of eltrombopag free acid to into the ethanolamine solution, keep warm and stir, cool down after the dropwise addition, filter to obtain a wet product, and dry the wet product to obtain Eltrombopag diethanolamine salt. Wherein, preferably, the ratio of the volume of tetrahydrofuran: mass of Eltrombopag free acid: volume of organic solvent is 6-11:1:12-24. The method comprises: dissolving ethanolamine in an organic solvent, raising the temperature to 60-75° C., stirring and dissolving to obtain a dissolved ethanolamine solution, and slowly adding the prepared eltrombopag free acid solution dropwise to the ethanolamine solution. Both tripopopag free acid solution and ethanolamine solution are clear...
Embodiment 1
[0041] Add 13.7g (0.22mol) of ethanolamine (analytical grade, the same below) and 235mL of ethanol (industrial grade, the same below) into a 500mL three-necked flask, stir magnetically, and heat up the oil bath to 65°C; Eltrombopag free acid (Ⅰ ) 10g (0.023mol) was added into 90mL of tetrahydrofuran (technical grade, the same below) and slightly heated (40°C) to dissolve, and the obtained solution of Eltrombopag free acid (I) was slowly added dropwise into the above-mentioned ethanol solution and kept stirring; After 2 hours of dripping, the temperature was lowered to 20-30°C in a water bath, suction filtered, and the filter cake was dried in a vacuum oven at 45°C for 8 hours to obtain 12.0 g of Eltrombopag diethanolamine (II), with a yield of 93.6%. GC detection solvent residual ethanol < 0.07%, tetrahydrofuran < 0.02%, the dissolved residue is qualified.
Embodiment 2
[0043] Add 13.7g (0.22mol) of ethanolamine and 235mL of ethanol to a 500mL three-necked flask, stir magnetically, and heat up the oil bath to 67°C; ℃) was dissolved, and the obtained solution of Eltrombopag free acid (I) was slowly added dropwise to the above-mentioned ethanol solution and kept stirring; after about 2 hours, the temperature was cooled to 20-30°C in a water bath, suction filtered, and the filter cake was put into Baked in a vacuum oven at 45° C. for 8 hours, and obtained 12.1 g of Eltrombopag diethanolamine (II), with a yield of 94.3%. GC detection of solvent residual ethanol <0.05%, tetrahydrofuran <0.01%, qualified for dissolution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com