Detection method of eltrombopag intermediate related substances and impurity reference substance used in method
A technology for the content of intermediates and impurities, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of Eltrombopag-free intermediate analysis methods, etc., and achieve convenient and specific research on impurity transfer rules and process parameter optimization , the effect of moderate analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
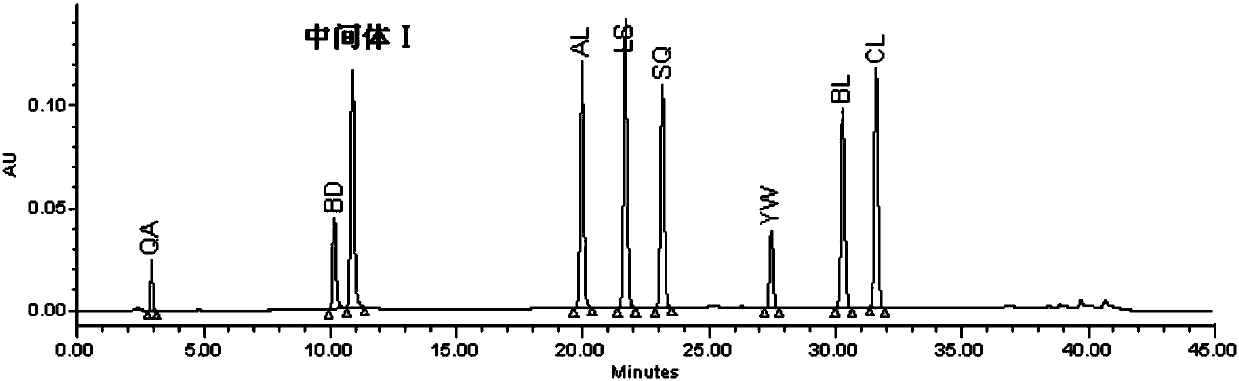
[0065] Embodiment 1 intermediate I method verification
[0066] 1. Chromatographic conditions
[0067] Instrument: Waters2695-2489-2998 high performance liquid chromatography - ultraviolet detector - diode array detector
[0068] Chromatographic column: use phenylsilane bonded silica gel [Xbridge Phenyl (4.6×250mm, 5μm)] as filler
[0069] Mobile phase A: 10mmol / L sodium dihydrogen phosphate (adjust the pH value to 3.5 with phosphoric acid)
[0070] Mobile phase B: 0.1% phosphoric acid in acetonitrile
[0071] gradient elution procedure
[0072] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
87
13
3
87
13
26
64
36
38
40
60
39
87
13
45
87
13
[0073] Flow rate: 1.2ml / min; Column temperature: 50°C; Detection wavelength: 230nm; Injection volume: 10μl
[0074] 2. Method verification
[0075] (1) System suitability
[0076] Impurity localization solution: Take the appropriate ...
Embodiment 2
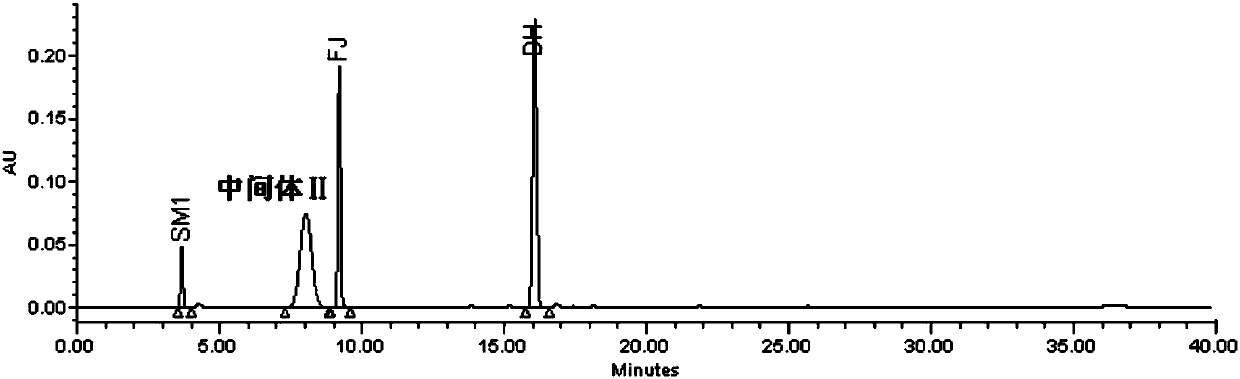
[0094] Embodiment 2 intermediate II method validation
[0095] 1. Chromatographic conditions
[0096] Instrument: Waters2695-2489-2998 high performance liquid chromatography - ultraviolet detector - diode array detector
[0097] Chromatographic column: use octadecylsilane bonded silica gel [Xbridge Shield RP-18 (4.6×150mm, 3.5μm)] as filler
[0098] Mobile phase A: 20mmol / L sodium dihydrogen phosphate (adjust the pH value to 3.0 with phosphoric acid)
[0099] Mobile Phase B: Acetonitrile
[0100] gradient elution procedure
[0101] time (min)
[0102] Flow rate: 1.0ml / min; Column temperature: 35°C; Sample chamber temperature: 4°C; Detection wavelength: 243nm; Injection volume: 10μl
[0103] 2. Method verification
[0104] (1) System suitability
[0105] Impurity localization solution: Take the appropriate amount of intermediate II, impurity SM1, impurity FJ, and impurity DH, weigh them accurately, put them in different measuring bottles, dissolve and dilute the...
Embodiment 3
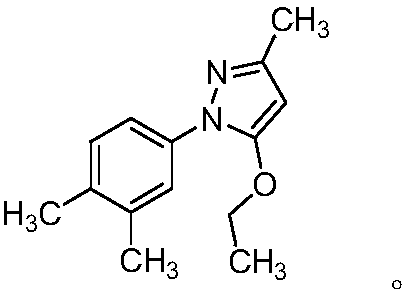
[0123] The preparation of embodiment 3 impurity compound DH
[0124]
[0125] Dissolve 3.0g of compound SM1, 2.4g of compound ethyl acetoacetate, and 3.6g of sodium bisulfite in 12mL of ethanol and 12ml of purified water, stir and heat up to reflux, and react for 3 hours; after the reaction is complete, cool down to 40°C and stir for 1 hour; Lower the temperature to 25°C, keep warm and crystallize for 5 hours; filter with suction, evaporate the filtrate to dryness, and separate by column chromatography to obtain 1.1 g of impurity DH.
[0126] ESI(+): m / z 231.20;
[0127] [M+1] + ; 1 H NMR: (400MHz,DMSO-d6)δ=7.40(d,1H),7.39(m,1H),7.13(d,1H),5.63(s,1H),4.10(d,2H),2.22(s ,3H), 2.19(s,3H), 2.12(s,3H), 1.31(m,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com