Method for verifying eltrombopag mediated anti-angiogenesis function of macrophage
An anti-angiogenesis and macrophage technology, applied in the field of verification of the anti-angiogenesis effect of macrophages mediated by Eltrombopag, can solve problems such as no related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
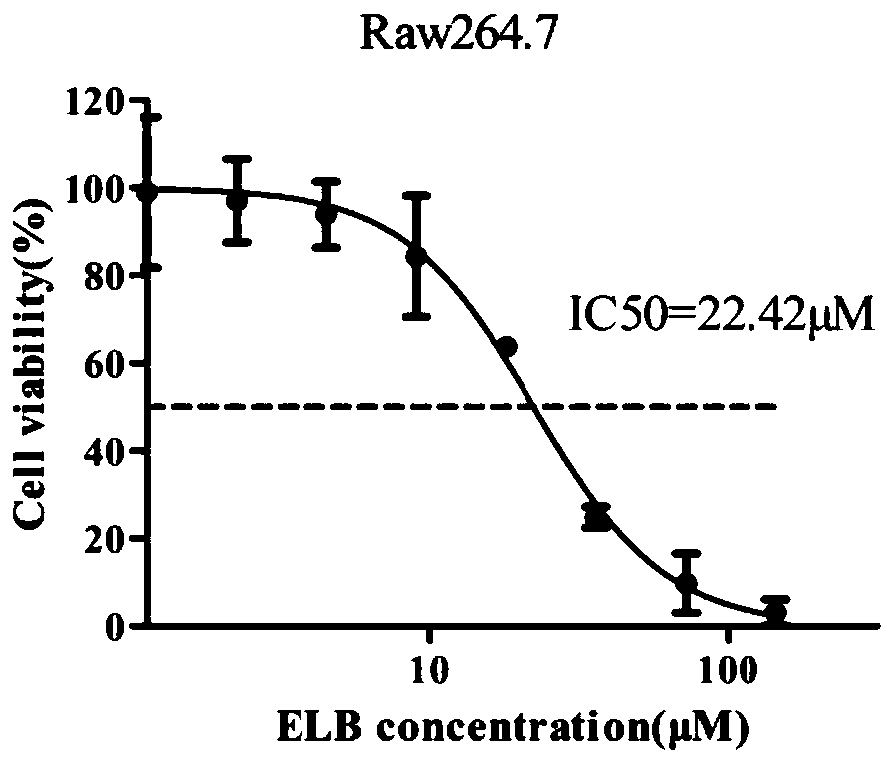
[0063] Example 1: MTT assay detects the effect of ELB on the proliferation of Raw264.7 cells
[0064] Take the Raw264.7 cells in the logarithmic growth phase, discard the old medium, wash with 3mL PBS, and discard the supernatant. Add 3mL PBS to each dish and pipette the cells repeatedly to make them fall off into a single cell suspension. Transfer the cell suspension into a 15mL centrifuge tube and centrifuge at 1000rpm for 5min.
[0065] Discard the supernatant, add an appropriate amount of complete culture medium, mix well, take a small amount of cell suspension for dilution, absorb 60 μL of the diluted liquid and add it to a cell counting plate for cell counting. Adjust the density of the cell suspension, inoculate 6,000 cells per well in a 96-well plate, add 100 μL per well, and add a circle of PBS to the outermost circle of the 96-well plate. Place the 96-well plate with cells in a 37°C, 5% CO2 cell incubator and culture overnight until it adheres to the wall. (The bl...
Embodiment 2
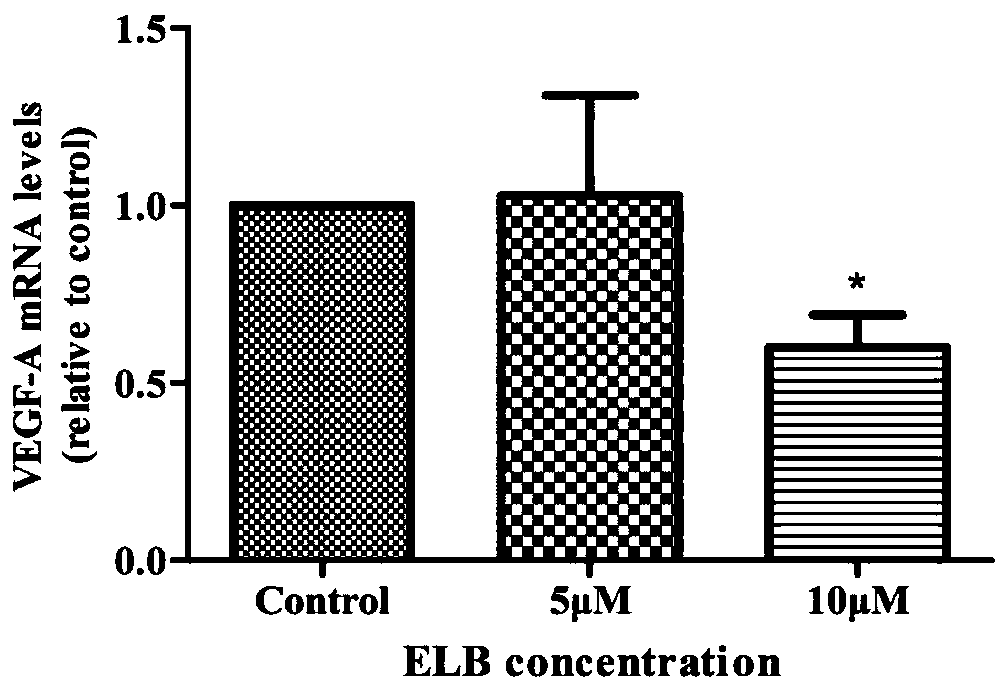
[0069] Example 2: Detection of mRNA expression of angiogenesis-related factors in Raw264.7 cells by q-PCR
[0070] Take the Raw264.7 cells in the logarithmic growth phase, discard the old medium, wash with 3mL PBS, and discard the supernatant. Add 3mL PBS to each dish and pipette the cells repeatedly to make them fall off into a single cell suspension. Transfer the cell suspension into a 15mL centrifuge tube. Centrifuge at 1000rpm for 5min.
[0071] Discard the supernatant, add an appropriate amount of PBS, mix well, take a small amount of cell suspension for dilution, absorb 60 μL of the diluted liquid and add it to a cell counting plate for cell counting. Adjust the cell suspension density to 1.5 x 10 6 Cells were seeded in 60 mm cell culture dishes, 2 mL per dish. Place the culture dish in a cell culture incubator for 3 h to make it adhere to the wall.
[0072] After 3 hours, the cell culture dishes were taken out, the supernatant was discarded, and 2 mL of RPMI medium...
Embodiment 3
[0074] Embodiment 3: ELISA detects the release of macrophage VEGF protein
[0075] Take the Raw264.7 cells in the logarithmic growth phase, discard the old medium, wash with 3mL PBS, and discard the supernatant. Add 3mL PBS to each dish and pipette the cells repeatedly to make them fall off into a single cell suspension. Transfer the cell suspension into a 15mL centrifuge tube. Centrifuge at 1000rpm for 5min.
[0076] Discard the supernatant, add an appropriate amount of RPMI 1640 and mix well, take a small amount of cell suspension for dilution, pipette 60 μL of the diluted liquid and add it to a cell counting plate for cell counting. Adjust the density of the cell suspension, inoculate every 12×106 cells in a 100mm cell culture dish, then replenish to 6mL with RPMI 1640, and put the culture dish into a 37°C, 5% CO2 incubator to balance for 12h.
[0077] After 12 hours, the culture dish was taken out, the supernatant was discarded, 4 mL of RPMI 1640 medium was added to eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com