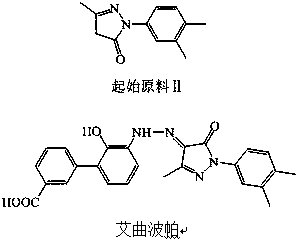
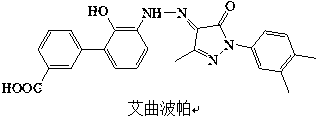
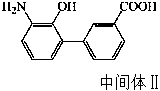
Preparation method of eltrombopag
A technology of intermediates and raw materials, which is applied in the field of Eltrombopag preparation, can solve the problems of long preparation process and difficult industrialization, and achieve the effects of strong operability, rapid response and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of Eltrombopag:
[0040] 1) Weigh 34.10g of starting material I, 400ml of glacial acetic acid and 200ml of hydrobromic acid (48%) into a 1L three-neck flask, start stirring, raise the temperature to 120°C, react for 3 hours, and stop the reaction. Cool down to 25°C, filter with suction, beat the filter cake with about 400ml of water, and dry at 55°C for 6 hours to obtain 30.7g of intermediate I as a yellow-green solid with a yield of 94.9% and a purity of 96.5%. 1 HNMR (400MHz, d 6 DMSO)δ13.90(s,1H),10.66(s,1H),8.12(t,J=1.7Hz,1H),
[0041] 8.07(dd, J=8.4,1.7Hz,1H),7.98(dt,7.8,1.5Hz,1H),7.74(dd,J=7.5,1.7Hz,1H),7.17(dd,J=8.4,7.5Hz ,1H).
[0042] 2) Weigh 30g of intermediate Ⅰ, 1.2g of Pd / C, and 150ml of water into a 500ml three-neck bottle, and add 150ml of hydrazine hydrate (98%) dropwise to the system. During this period, heat and gas are released violently, and the temperature is controlled at 25°C. After the dropwise addition, the temperatur...
Embodiment 2
[0046] The preparation method of Eltrombopag:
[0047] 1) Weigh 341g of starting material I, 4L of glacial acetic acid and 2L of hydrobromic acid (48%) into a 1L three-necked flask, start stirring, raise the temperature to 120°C, and stop the reaction after 3 hours of reaction. Cool down to 25°C, filter with suction, beat the filter cake with about 4L of water, and dry at 55°C for 6 hours to obtain 310 g of yellow-green solid intermediate Ⅰ, with a yield of 95.8% and a purity of 96.7%.
[0048] 1 HNMR (400MHz, d 6 DMSO)δ13.90(s,1H),10.66(s,1H),8.12(t,J=1.7Hz,1H),8.07
[0049] (dd, J=8.4,1.7Hz,1H),7.98(dt,7.8,1.5Hz,1H),7.74(d d,J=7.5,1.7Hz,1H),7.17
[0050] (dd, J=8.4, 7.5Hz, 1H).
[0051] 2) Weigh 300g of intermediate Ⅰ, 6gPd / C, and 1.5L of water into a 5L three-neck bottle, and add 1.5L of hydrazine hydrate (98%) dropwise to the system. During this period, heat and gas are released violently, and the temperature is controlled at 25°C. After the dropwise addition, the tem...
Embodiment 3
[0055] The preparation method of Eltrombopag:
[0056] 1) Weigh 3.41g of starting material I, 40ml of glacial acetic acid and 20ml of 48% hydrobromic acid (48%) into a 100ml three-neck flask, start stirring, raise the temperature to 120°C, and stop the reaction after 3 hours of reaction. Cool down to 25°C, filter with suction, beat the filter cake with about 40ml of water and filter with suction, and dry at 55°C for 6 hours to produce 3.10g of yellow-green solid intermediate Ⅰ, yield: 92.7%, purity: 96.1%. 1 HNMR (400MHz, d 6 DMSO)δ13.9(s,1H),10.66(s,1H),8.12(t,J=1.7Hz,1H),
[0057] 8.07(dd,J=8.4,1.7Hz,1H), 7.98(dt,7.8,1.5Hz,1H),7.74(dd,J=7.5,1.7Hz,1H)
[0058] ,7.17(dd,J=8.4,7.5Hz,1H).
[0059] 2) Weigh 3g of intermediate I, 0.15g of Pd / C, and 15ml of water into a 100ml three-necked flask, and add 15ml of 98% hydrazine hydrate dropwise to the system. During this period, heat and gas are released violently, and the temperature is controlled at 25°C. After the dropwise addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com