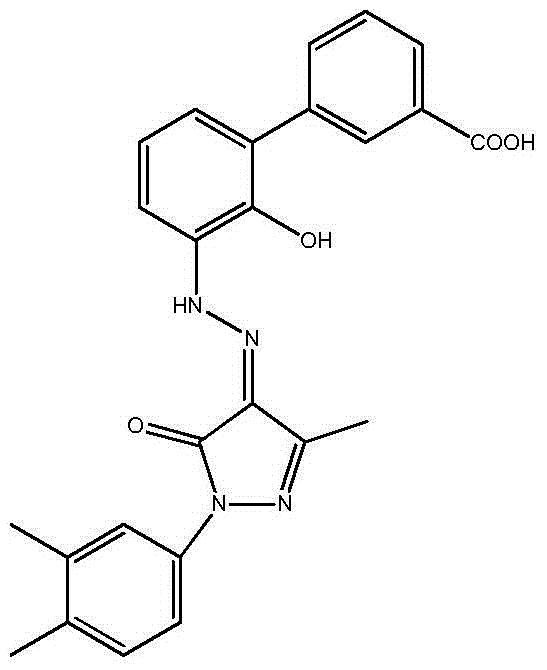
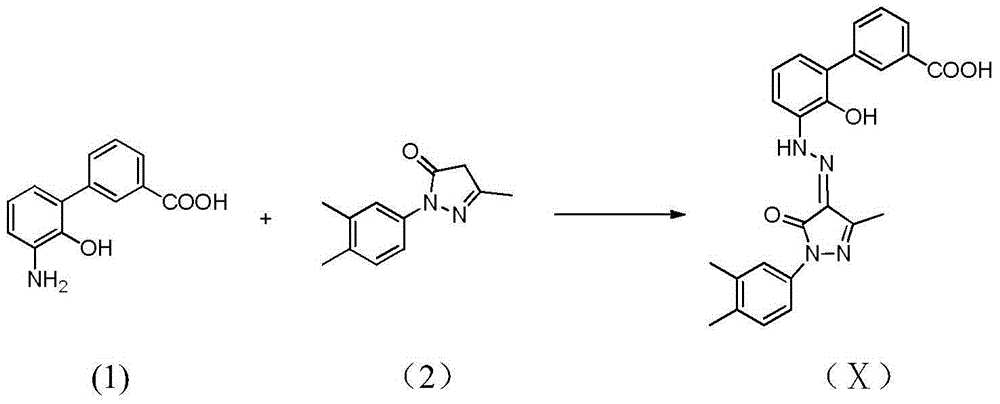
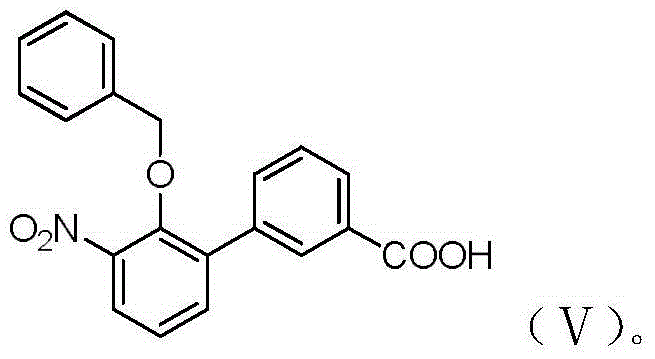
Eltrombopag intermediate and preparation method therefor and application thereof
An Eltrombopag and reaction technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as many steps, unsuitable for large-scale industrial production, etc., and achieve a simple preparation method and high safety. , cost saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0027] Example 1-3: Preparation of 2-benzyloxy-1-bromo-3-nitrobenzene
[0028] 10.9g (50mmol) of 2-nitro-6-bromophenol, 9g (52mmol) of benzyl bromide, 7.6g (55mmol) of potassium carbonate were mixed in acetonitrile (120ml) and reacted under reflux for 3 hours until the reaction is complete. Cool to room temperature and filter Concentrate to dryness to obtain a concentrate, add ethyl acetate (100ml) to dissolve, wash with water (50ml), saturated brine (50ml), dry, and concentrate to dryness to obtain 15g of yellow crystals with a yield of 97.4%.
[0029] Mix 2-nitro-6-bromophenol (4.36g), benzyl bromide (3.6g), potassium carbonate (4.14g) and tetrahydrofuran (50ml), stir under reflux for 3 hours, cool, filter, and concentrate the filtrate to dryness. Add ethyl acetate (30ml) and water (50ml), separate the layers, extract the aqueous layer with ethyl acetate (30ml×2), combine the organic layers, wash with water, washed with saturated brine, dry, filter, and concentrate to obtain the ...
Embodiment 4-5
[0031] Example 4-5: Preparation of 3'-nitro-2'-benzyloxy-[1,1'-biphenyl]-3-carboxylic acid
[0032] The compound obtained in Example 1 was 12.32g (40mmol), 3-carboxyphenylboronic acid 7.97g (48mmol), potassium carbonate 8.28g (60mmol), [1,1'-bis(diphenylphosphine)ferrocene] Palladium dichloride 2.04g (2.8mmol), mixed in 1,4-dioxane (200ml) and water (40ml) at 60°C under nitrogen protection for 4 hours, cooled and filtered, and concentrated to remove 1,4-dioxane Ring, add water (100ml), adjust the pH to acidity with 1mol / L dilute hydrochloric acid, a solid precipitated out, and filtered to obtain 14g of a dark brown solid. It was recrystallized with isopropanol (60ml) and water (20ml), and finally 11.5g of light brown solid was obtained with a yield of 82.4%. 1 HNMR(400MHz,CDCl3)δ8.30(s,1H), 8.18(d,J=7.7Hz,1H), 7.85(d,J=7.7Hz,1H), 7.81(dd,J=8.1,1.4Hz, 1H), 7.62(dd,J=7.6,1.3Hz,1H), 7.55(t,J=7.7Hz,1H), 7.33(t,J=7.9Hz,1H), 7.26–7.20(m,3H), 7.05(dd,J=6.6,2.5Hz,2H),4.66(s,2H).ESI-MS(...
Embodiment 6-7
[0034] Example 6-7: Preparation of 3'-amino-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid
[0035] Dissolve 10.47g (30mmol) of the compound obtained in Example 4 in ethyl acetate (350ml), add 1.05g of 10% palladium on carbon, react with hydrogen under pressure at 50°C for 10 hours, cool, filter, and concentrate to dryness to obtain yellow Solid 6.6g. The yield was 96%. 1 HNMR(400MHz,DMSO-d 6 )δ8.08(s,1H),7.93(d,J=7.7Hz,1H), 7.73(d,J=7.8Hz,1H), 7.58(t,J=7.7Hz,1H),7.44(dd, J=7.8Hz,1H),7.26(dd,J=7.7,1.4Hz,1H),7.04(t,J=7.8Hz,1H).ESI-MS(m / z):228[MH] -
[0036] Dissolve 10.47g (30mmol) of the compound obtained in Example 4 in ethyl acetate (350ml), add 2.1g of 10% palladium on carbon, and react with hydrogen under pressure at 50°C for 8 hours, cool, filter, and concentrate to dryness to obtain yellow Solid 6.7g. The yield was 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com