Improved anticancer treatments
A technology for cancer treatment and anticancer drugs, which is applied in the direction of antineoplastic drugs, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as narrow therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
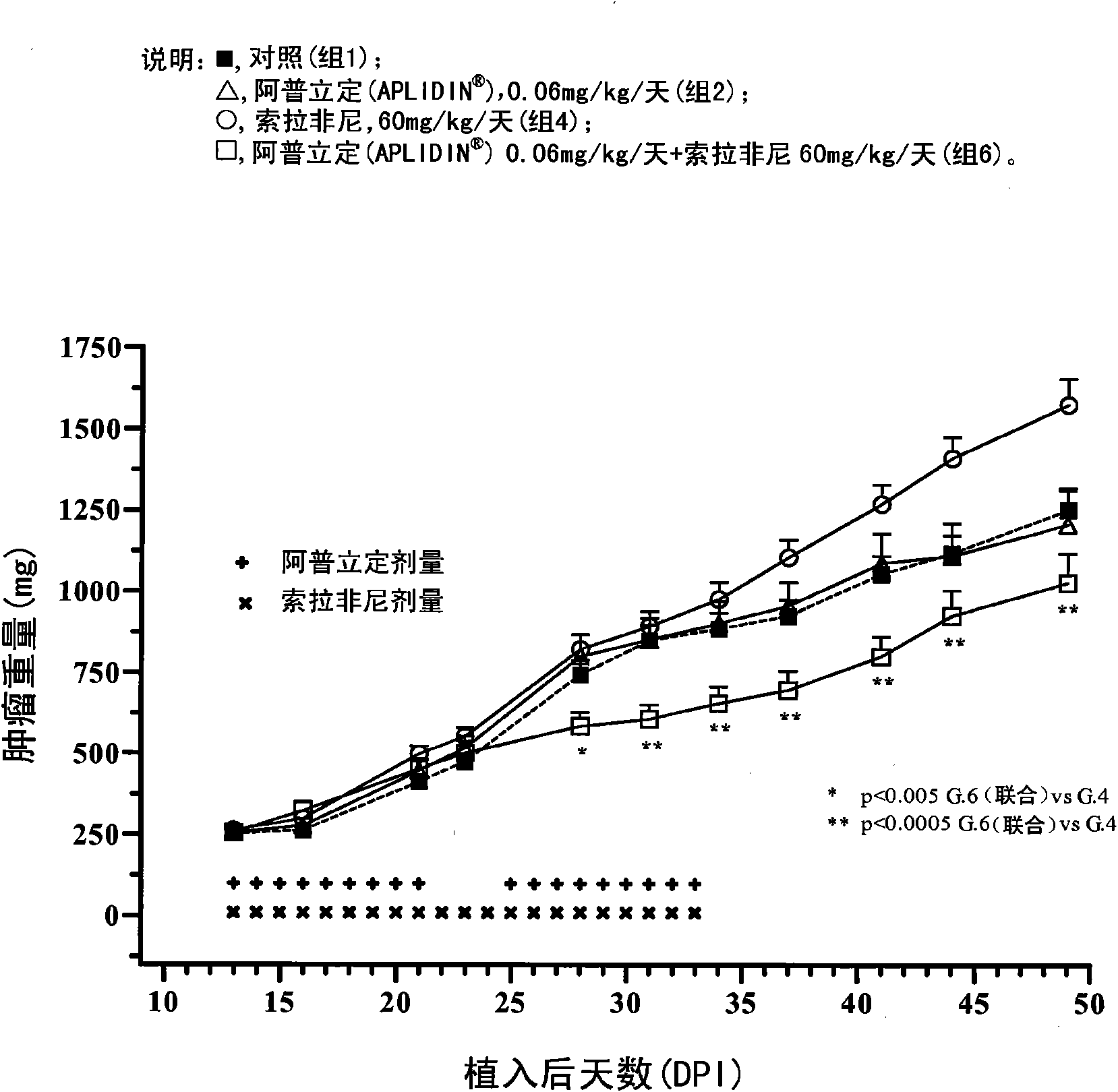
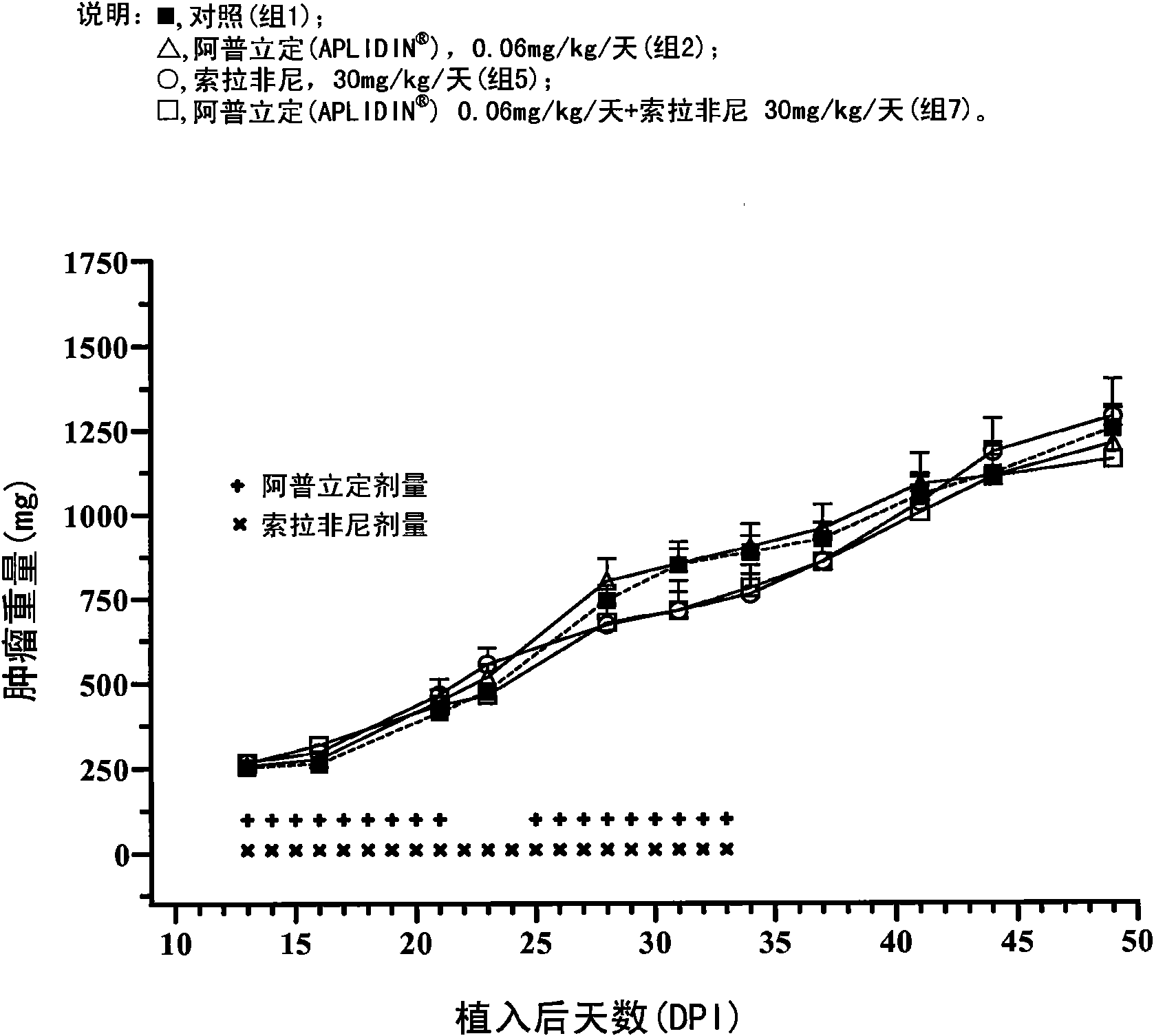
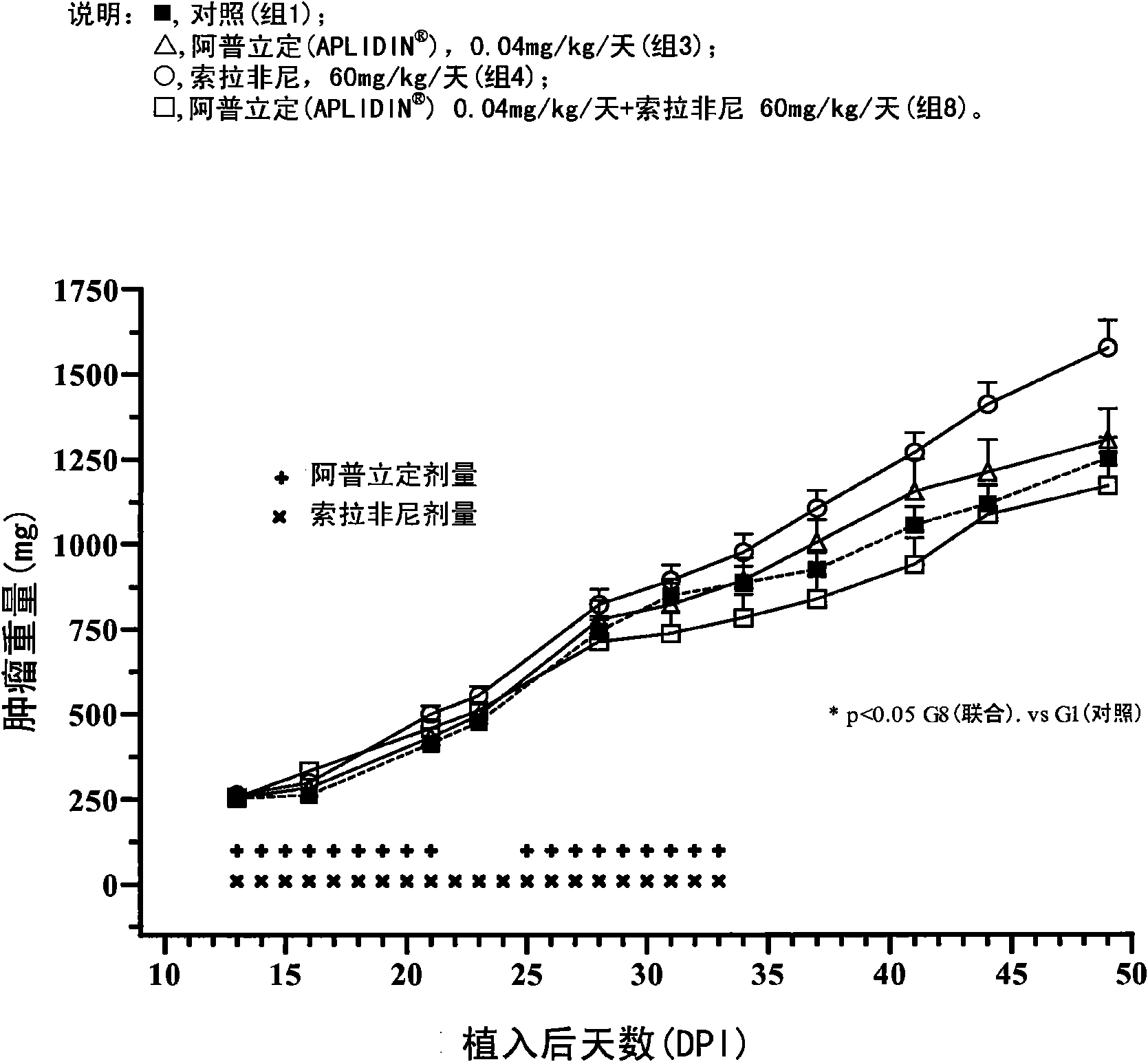
[0149] Example 1 In vivo study to determine the effect of aplidine in combination with sorafenib in human renal tumor xenografts.
[0150] The goal of the study was to assess the ability of aplidine to potentiate the antitumor activity of sorafenib by using three xenograft models of human renal carcinoma.
[0151] Female athymic nude mice (Harlan Sprague Dawley, Madison, WI) were used in all experiments. When the mice were 5 weeks old, tumor fragments or cell suspensions were implanted into the mice. Animals were housed in ventilated rack cages, 5 mice per cage, with food and water ad libitum. Mice were acclimated for 1 week prior to implantation with tumor fragments or cell suspension. The vehicle control group contained 15 mice, and the treatment groups had 10 mice each.
[0152] The tumor models used in this study were the CAKI-1 cell line, which is a human renal clear carcinoma cell line from ATCC (Manassas, VA), the MRI-H-121 cell line, and the A498 cell line, so The ...
Embodiment 3
[0304] Example 3 In vivo study to determine the effect of the combination of aplidine and temsirolimus on human renal tumor xenografts.
[0305] The goal of this study was to assess the ability of aplidine to potentiate the antitumor activity of temsirolimus by using a xenograft model of human renal carcinoma.
[0306] Female athymic nude mice (Harlan Sprague Dawley, Madison, WI) were used in all experiments. Tumor fragments were implanted into mice when they were 5 weeks old. Animals were housed in ventilated rack cages, 5 mice per cage, with food and water provided ad libitum. Mice were acclimated for 1 week prior to implantation with tumor fragments. The vehicle control group contained 15 mice, and the treatment groups had 10 mice each.
[0307] The tumor model used for the study was the MRI-H-121 cell line, which is a human renal carcinoma cell line from the DCT tumor bank. For the MRI-H-121 test, use a trocar to place the 3mm 3 Tissue tumor fragments from an in viv...
Embodiment 4
[0333] Example 4 In vivo study of aplidine combined with temsirolimus for CAKI-1 therapy in human renal tumor xenografts.
[0334] Female athymic nude mice (Harlan Sprague Dawley, Madison, WI) were used in all experiments. The cell suspension was implanted into the mice when they were 6-8 weeks old. Animals were housed in ventilated rack cages with food and water provided ad libitum. Mice were acclimatized for at least 5 days prior to tumor implantation. The vehicle control group contained 15 mice, and the treatment groups had 10 mice each. The tumor model used for this study was CAKI-1, which was implanted into animals as disclosed in Example 2.
[0335] Tumor size was determined as disclosed in Example 1. When tumors grew within a mean size range of 140±34 mg (mean±SD), according to the Tumor Weights Determined by Life Module V 8.0 SP1 Tumor Tracking and Measurement Software Mice were randomized into treatment and control groups.
[0336] Treatment of CAKI-1 tumor-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com