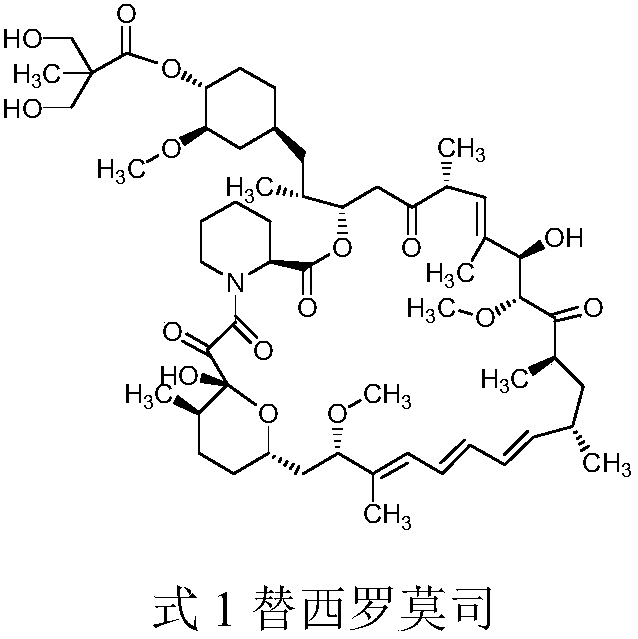
Method for purifying temsirolimus
A technology of temsirolimus and purification method, applied in the direction of organic chemistry, etc., can solve the problems of being unsuitable for industrial production, unable to meet industrialization requirements, and high cost of high-efficiency liquid phase systems, and achieves small complexes, mild conditions, and processes. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 200g of 200-300mesh silica gel, soak it in petroleum ether and put it into a chromatography column for use; weigh 30g of temsirolimus crude product (purity 85.4%, maximum simple impurity 6.2%), dissolve it in 120ml ethyl acetate, load , after loading the sample, use 1800ml volume ratio of 1:1, 1:2, 2:5 mixed solution of petroleum ether and ethyl acetate to elute the impurities, then use ethyl acetate to elute and collect Division of the solution; maintain 20 ~ 25 ° C, the vacuum degree is not lower than -0.09Mpa conditions under reduced pressure to concentrate the collected solution to obtain 24.6 g of light yellow foamy solid. In a 500ml three-necked flask, add 24.6g of light yellow solid and 148ml of ethyl acetate obtained above, heat up to 30°C, stir to dissolve, add 2.5g of activated carbon, continue stirring for 30min and filter; transfer the filtrate into a 1000ml three-necked flask, stir slowly and drop Add 248ml of petroleum ether, slowly cool down to -10~...
Embodiment 2
[0024] Weigh 200g of 200-300 mesh silica gel, soak it with petroleum ether and put it into a chromatography column for use; weigh 30g of temsirolimus crude product (purity 85.4%, maximum simple impurity 6.2%) and dissolve it in 120ml dichloromethane, load the sample , after loading the sample, use 1800ml volume ratio of 1:1, 1:2, 2:5 mixed solution of petroleum ether and ethyl acetate to elute the impurities, then use ethyl acetate to elute and collect Division of the solution; maintain 20 ~ 25 ° C, the vacuum degree is not lower than -0.09Mpa conditions under reduced pressure to concentrate the collected solution to obtain 25.4 g light yellow foamy solid. In a 500ml three-necked flask, add 25.4g of the light yellow solid obtained above and 203ml of acetone, heat up to 25°C, stir to dissolve, add 2.6g of activated carbon, continue to stir for 30min and filter; transfer the filtrate to a 1000ml three-necked flask, stir and slowly add 305ml n-hexane, slowly lower the temperature...
Embodiment 3
[0026] Weigh 200g of 200-300 mesh silica gel, soak it with petroleum ether and put it into a chromatography column for use; weigh 30g of temsirolimus crude product (purity 85.4%, maximum simple impurity 6.2%) and dissolve it in 120ml dichloromethane, load the sample , after loading the sample, use 1800ml volume ratio of 1:1, 1:2, 2:5 mixed solution of petroleum ether and ethyl acetate to elute the impurities, then use ethyl acetate to elute and collect Division of the solution; maintain 20 ~ 25 ° C, the vacuum degree is not lower than -0.09Mpa conditions under reduced pressure to concentrate the collected solution to obtain 25.1 g light yellow foamy solid. In a 500ml three-necked flask, add 25.1g of the light yellow solid obtained above and 151ml of ethanol, heat up to 30°C, stir to dissolve, add 2.5g of activated carbon, continue to stir for 30min and filter; transfer the filtrate to a 1000ml three-necked flask, stir and slowly add 376ml Heptane, slowly cool down to -10~-5°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com