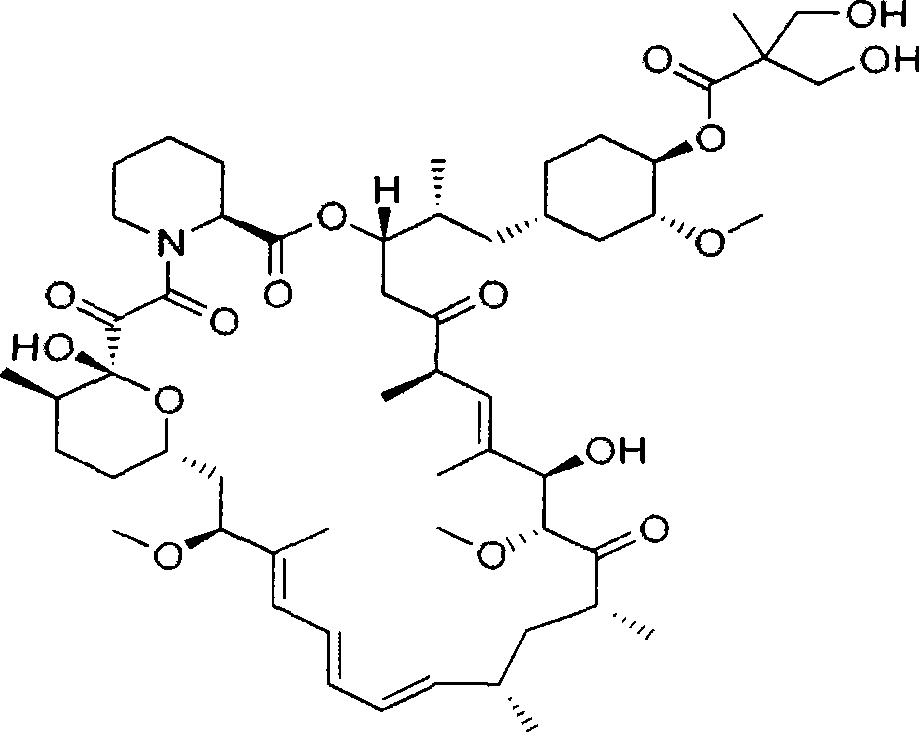
Medicinal composition containing temsirolimus and preparation method of medicinal composition
A technology of composition and esterification, which is applied in the field of pharmaceutical composition containing temsirolimus ester compound and its preparation, can solve problems such as hypersensitivity, increased irritation or toxicity, and vascular atrophy, so as to reduce production costs, Improved stability and process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
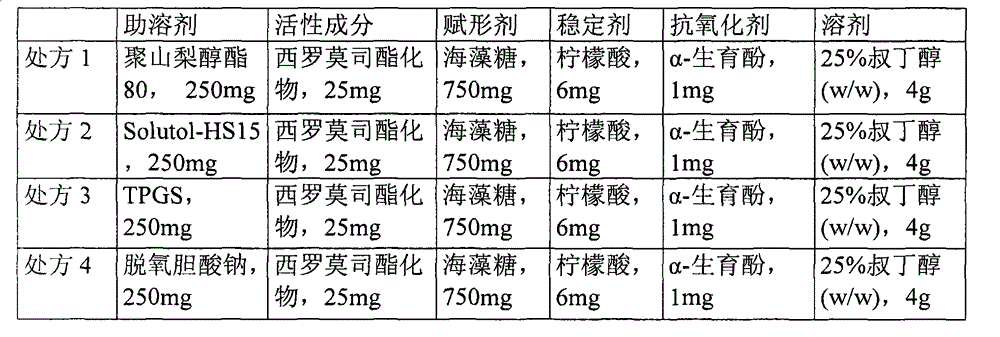
[0028] Because the water solubility of temsirolimus ester compound is very poor, solubilizer is needed to solubilize, the contriver is to commonly used cosolvent polysorbate 80 in the injection, polyethylene glycol stearate 15 (Solutol-HS15), Tocopheryl polyethylene glycol succinate (TPGS), sodium deoxycholate, etc. were screened, and the prescription was designed as follows:
[0029] Co-solvent screening of temsirolimus ester lyophilized preparation
[0030]
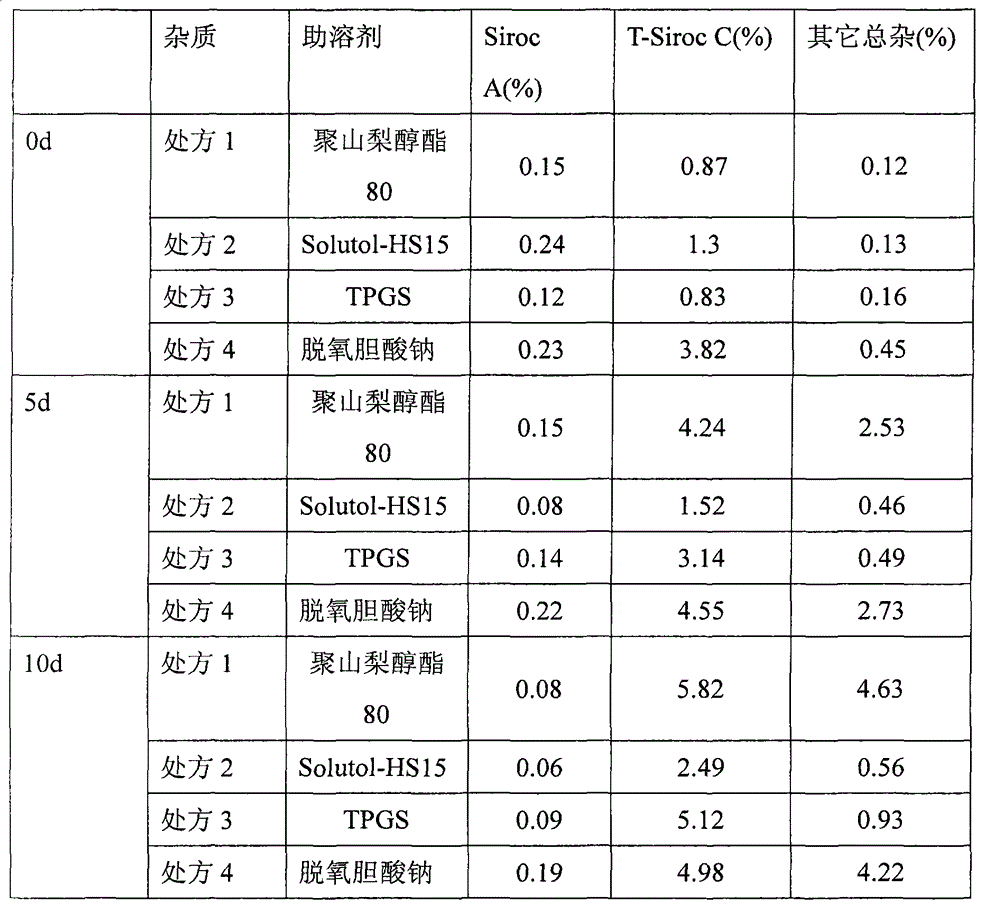
[0031] The above prescription was studied on the influencing factors at 40°C, and the results are shown in the table below:
[0032]
[0033] The experimental results show that in four typical co-solvents polysorbate 80, polyethylene glycol stearate 15 (Solutol-HS15), tocopherol polyethylene glycol succinate (TPGS), sodium deoxycholate , the stability of the pharmaceutical composition containing the temsirolimus ester compound of polyethylene glycol stearate 15 (Solutol-HS15) is the best, so in follow-up research, ...
Embodiment 2
[0035] according to Formulation of Concentrate A concentrate of temsirolimus ester was prepared as a control. outsourcing A concentrate formulation served as a control. Concentrate Prescription
[0036]
[0037] Dissolving the prescribed amount of active ingredients sirolimus ester, anhydrous citric acid, and d,l-alpha-tocopherol in a composite solvent of absolute ethanol and propylene glycol, filling according to the content, filling with nitrogen, and capping. The sirolimus ester concentrate was used as a control.
Embodiment 3
[0039] Prescription of temsirolimus ester freeze-dried pharmaceutical composition
[0040] Component role
ingredients
Dosage
sirolimus ester
25mg
750mg
stabilizer
0.25mg
Antioxidants
0.75mg
Co-solvent
Solutol-HS15
250mg
solvent
Solvents used in the process and eventually removed
water and tert-butanol
[0041] Dissolve the active ingredient temsirolimus ester compound, anhydrous citric acid and α-tocopherol in the prescribed amount in tert-butanol to make the oil phase, dissolve Solutol-HS15 and trehalose in water to make the water phase, and the water phase oil After the phases were mixed, the volume was adjusted to 5 g, and then stirred to clarify to obtain a freeze-dried stock solution.
[0042] After the above preparation was freeze-dried, filled with nitrogen, capped, and stored at 25°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com