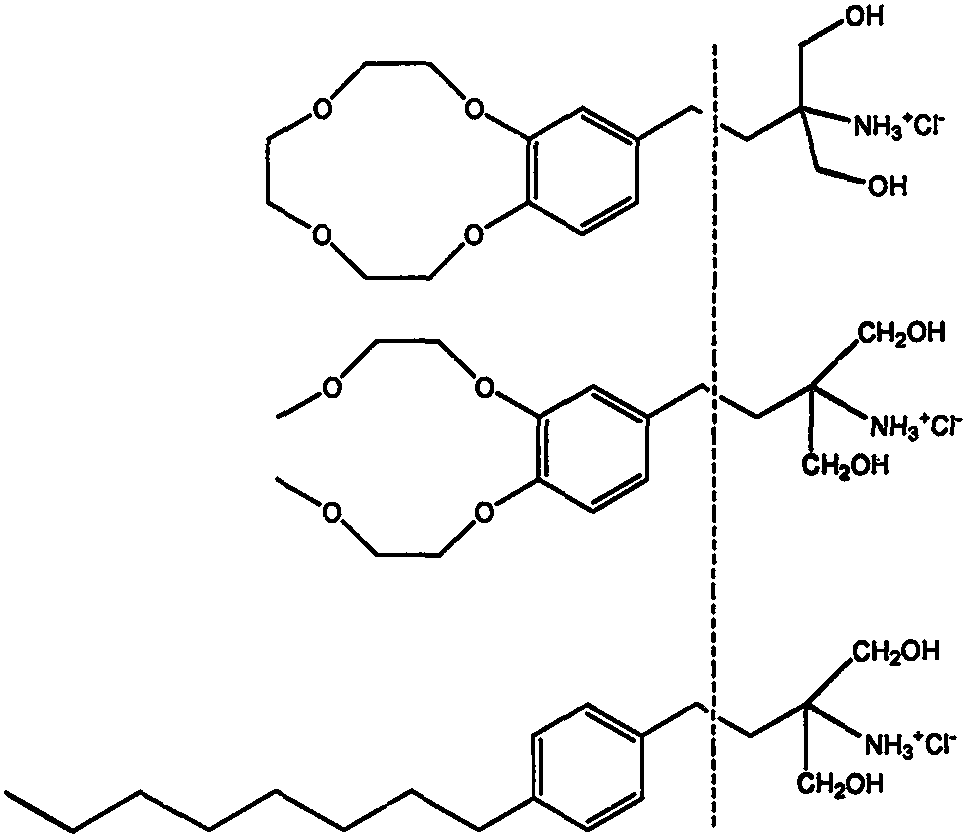
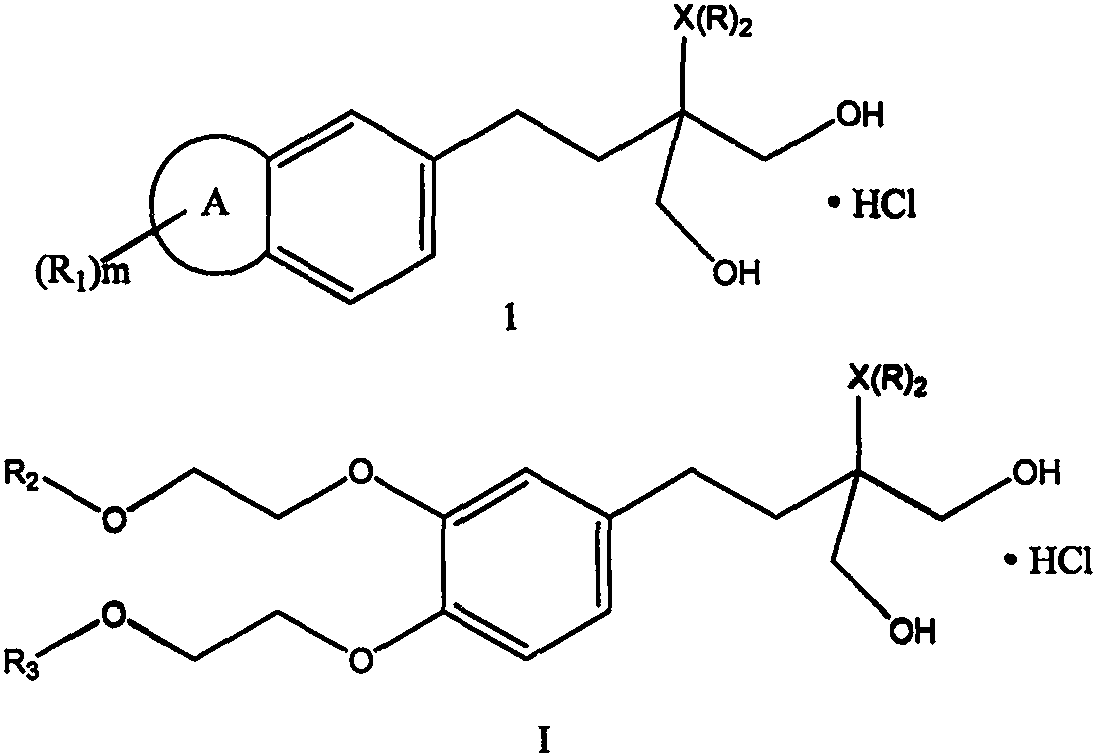
Fingolimod derivative containing crown ether and di (2-methoxyethoxy) structure
A technology of structural formula and alkoxy group, which is applied in the field of drug synthesis and can solve problems such as limiting the application of the treatment of immune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
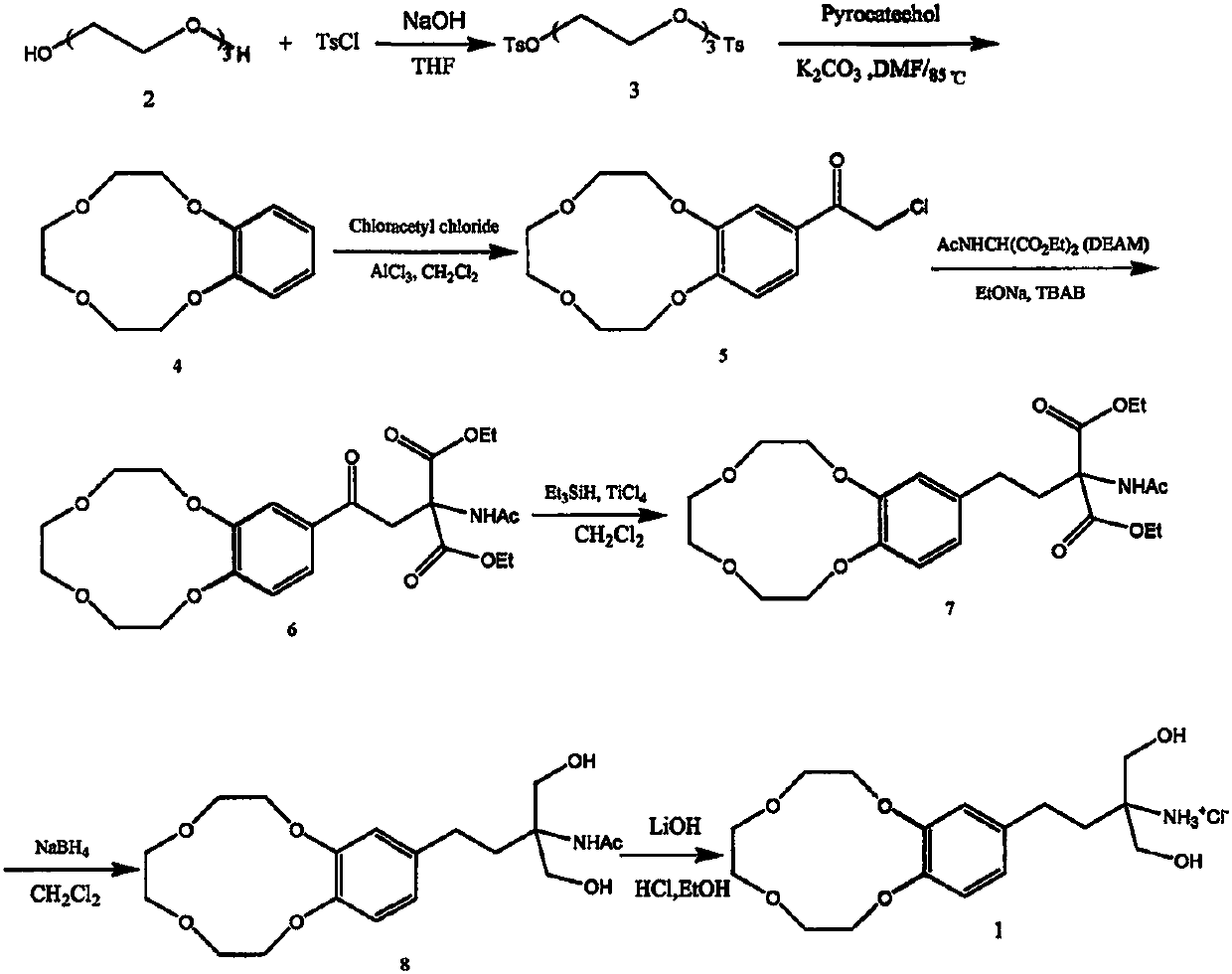
Embodiment 1
[0032] Preparation of 1,8-bis(p-toluenesulfonate)-3,6-dioxoctane (2)
[0033] Add 16kg (400mol) sodium hydroxide and 80L water into the 400 reaction kettle to dissolve, then add 18.8L (140mol) triethylene glycol and 32L tetrahydrofuran into the reaction kettle, cool below 5°C, and drop 47.84kg (260mol ) p-toluenesulfonyl chloride, 50L tetrahydrofuran solution, after dropping, react at this temperature for 2h, pour into 240L ice water, filter with suction, wash with a small amount of water, and dry to obtain 58.64kg white crystalline powder with a yield of 91.4%. mp: 77-80°C, HPLC: 97%. TLC (petroleum ether:ethyl acetate=1:1, Rf=0.87). 1 HNMR (CDCl 3 ): δppm: 7.78 (d, 4H, J=10.4Hz, the benzene ring relies on the sulfonyl proton); 7.34 (d, 4H, J=11.6Hz, the benzene ring relies on the methyl proton); 4.129 (dd, 4H, J= 5.6Hz, close to the sulfonyl glycol proton); 3.64 (dd, 4H, J=5.6Hz, away from the sulfonyl glycol proton); 3.517 (s, 4H, a molecule of ethylene glycol proton in ...
Embodiment 2
[0035] Preparation of 3,4-benzo-12-crown-4-benzene (3)
[0036] Mix 2.2kg (20mol) catechol, 12.4kg (89.6mol) potassium carbonate and 300L DMF, stir for about 30min., heat up to 85-90°C, add 8-di(p-toluenesulfonate)-3 dropwise, Preparation of 6-dioxoctane (2) 40L of DMF solution of 9.17kg (20mol), dripping is finished within 1.5~2h, dripping is finished, reaction is 30min., TLC checks that reaction is complete (developing agent: sherwood oil: ethyl acetate = 1:1, Rf = 0.58). Suck out about 40L of the reaction solution, continue to repeat the above operation 3 to 5 times, filter with suction, evaporate the DMF to dryness under reduced pressure, dissolve the residue with 240L of ethyl acetate, filter with suction, evaporate under reduced pressure, and use petroleum ether:ethyl acetate for the residue= 1:1 for column chromatography. The solvent was recovered under reduced pressure, and the solid was recrystallized with isopropanol 1:2.5 (W / V) to obtain 1.376 kg of off-white powd...
Embodiment 3
[0043] 3. Preparation of 4-benzo-12-crown-α-chloroacetophenone (4)
[0044] Under ice cooling (0°C), 3,4-benzo-12-crown-4-benzene (18 g, 80.3 mmol) was dissolved in 200 mL of dry CH 2 Cl 2 In, add chloroacetyl chloride (9.1g, 80.3mmol), then slowly add AlCl 3 (16.1g, 120.5mmol), when AlCl 3 After all was added, it was naturally raised to room temperature and continued to stir for 2h. TLC (petroleum ether: ethyl acetate = 1:1, Rf = 0.52) detected that the raw material point disappeared, and the reaction solution was poured into ice water hydrochloric acid to decompose, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extracted 3 times, combined organic layers, washed to neutral, anhydrous Na 2 SO 4 After drying, filtering and concentrating, 22.6 g of the crude product was obtained as a pale yellow solid. , suction filtration, and ethanol recrystallization to obtain 19.2 g of slightly yellow to yellow crystalline powder, with a yield of 79%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com