High-activity exendin-4 analogue and pharmaceutical application thereof
A technology of exenatide and analogs, which is applied in the fields of exenatide analogs and medical applications to achieve the effects of treating or reducing diabetes and obesity, inhibiting gastrointestinal motility and lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Design of Exenatide Analogues
[0041] Design 1: Taking the binding energy of exenatide analogues to GLP-1 receptor as the inspection index, and taking the crystal structure of exenatide binding to GLP-1 receptor (PDBID: 3C5T) (JBiolChem.283(2008)11340) –11347) as the object, using FoldX software (JMolBiol.320(2002)369–387; ProcNatlAcadSciUSA.102(2005)10147–10152) to design, and get several candidate mutants with improved binding energy, including EX-E16W(SEQIDNo :2), EX-R20M (SEQIDNo:16), EX-K27M (SEQIDNo:17) and EX-G29W (SEQIDNo:9).
[0042] Design 2: Because the stability of the α-helical region in the middle of the exenatide molecule has a certain positive correlation with its activity (Biochemistry. 46 (2007) 5830-5840). The design idea is to introduce more intra-chain ionic bonds into the exenatide α-helix to improve its activity. After analyzing the three-dimensional structure of Exenatide, the inventors found that the glutamic acid at positions Leu21 and ...
Embodiment 2
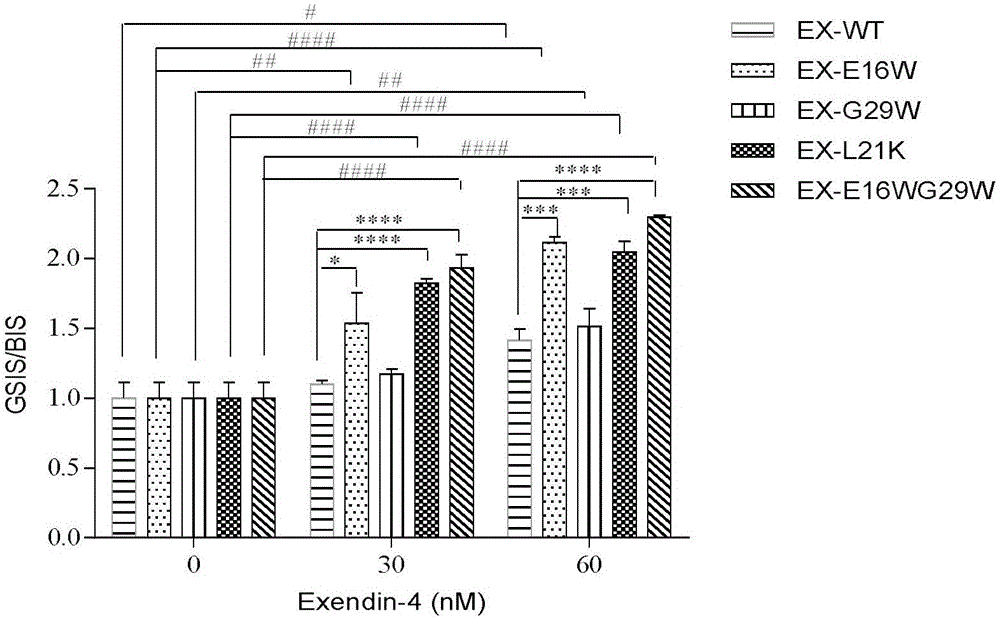
[0045] Example 2 Screening of exenatide analogues in vitro to stimulate insulin secretion activity of rat islet cell tumor cells INS-1
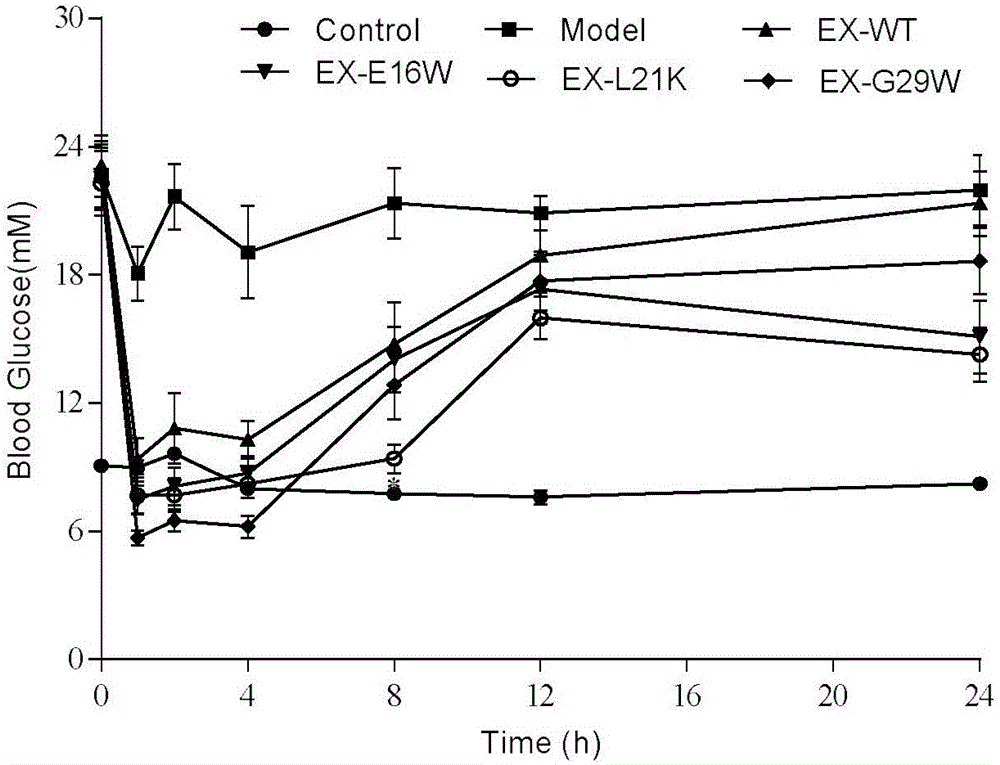
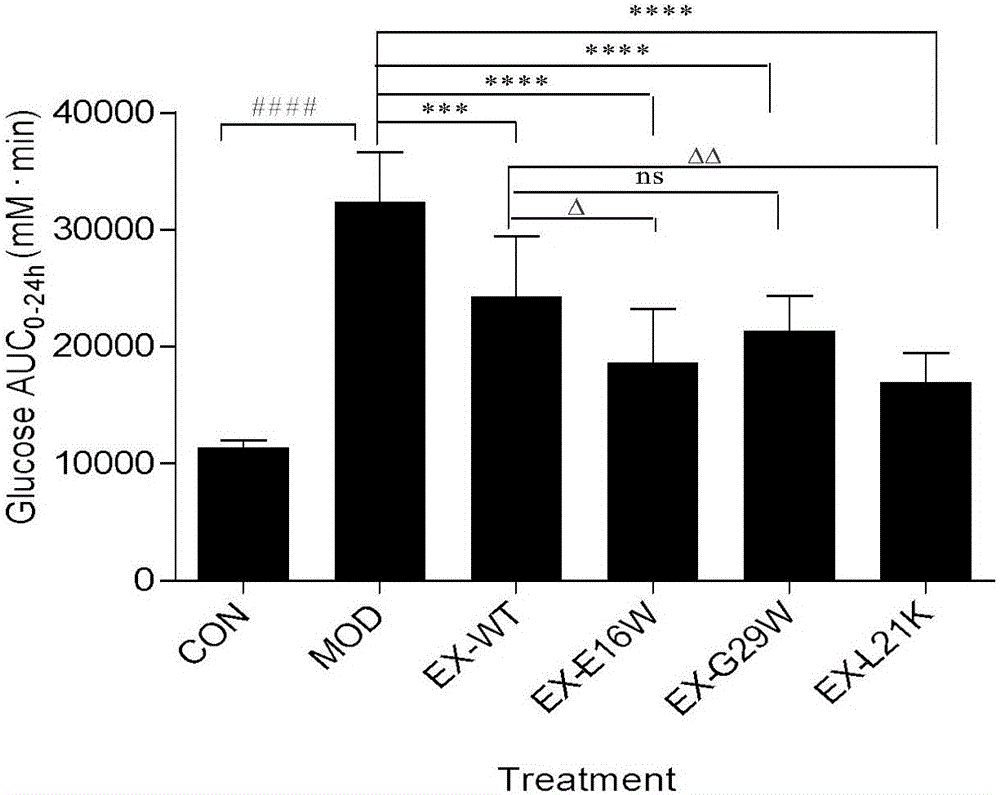
[0046] Rat islet cell tumor cells INS-1 were cultured with RPMI-1640 medium to about 80% confluence, discarded the stock solution, washed twice with PBS buffer, digested with 0.05% trypsin at 37°C for 2 minutes, and used RPMI- containing 10% FBS. The digestion was terminated by 1640, and the cells were collected by centrifugation at 800 rpm for 5 minutes, resuspended in cell culture medium, and then resuspended at 5×10 5 Cells / well are seeded on a 12-well cell culture plate, placed at 37℃, 5% CO 2 Incubate in an incubator for 24 hours, discard the stock solution, and wash once with PBS for experimentation. Experimental grouping: 1. Basal insulin secretion group (containing 0, 30, 60 nM) Exenatide and the serum-free medium of similar drugs designed in Example 1 of the present invention were treated for 24 hours, and each drug concentration was set...
Embodiment 4
[0073] MOE software (Molecular Operating Environment, Chemical Computing Group, Montreal, Canada) was used to analyze the differences between the interactions between mutant EX-E16W, EX-G29W and wild-type exenatide EX-WT and GLP-1 receptor.
[0074] The results showed that, compared with the wild type, the number of interactions between the mutant EX-E16W and EX-G29W and the receptor increased, that is, a new interaction was formed between the mutation site and the receptor.
[0075] Image 6 It is a schematic diagram of the interaction between Glu at position 16 of wild-type Exenatide EX-WT and the receptor. Figure 7 It is a schematic diagram of the interaction between Gly at position 29 of wild-type Exenatide EX-WT and the receptor. Image 6 with Figure 7 It shows that there is no interaction between Glu at position 16 and Gly at position 29 of wild-type exenatide EX-WT and GLP-1 receptor.
[0076] However, in the mutant EX-E16W, the mutated Trp16 forms two new hydrophobic interac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com