Extract of traditional Chinese medicine having alpha-glucosidase inhibitor activity and its application
A technology of glycosidase inhibitors and extracts, which is applied to traditional Chinese medicine extracts with α-glucosidase inhibitor activity and its application fields, and can solve problems such as no involvement of traditional Chinese medicine extracts, no involvement, unclear active ingredients and mechanism of action, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment one, the preparation of extract
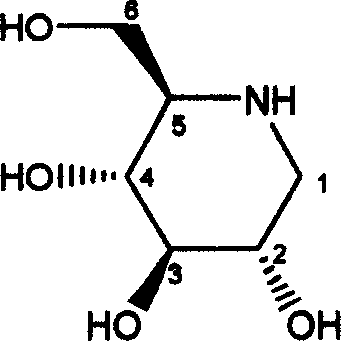
[0016] Dried 500 g of Guangdong mulberry leaf fine powder, extracted with 4 L of 60% ethanol under reflux for 2 hours, and filtered. Add 3 L of 60% ethanol to the filter residue for reflux extraction for 2 hours, and filter. Combine the extracts, recover until there is no ethanol smell, add an equal volume of ethanol, and centrifuge to remove the precipitate. The supernatant is passed through 732 (H+ type) strong acid ion exchange resin, and eluted with 2L of water and 4L of 0.5N ammonia water respectively. The ammonia water part is concentrated and dried 2g ninhydrin reaction is positive containing more than 10% cytidine, 2-(1', 2', 3', 4'-tetrahydroxybutyl)-5-(2", 3", 4"- Total alkaloid extract of trihydroxybutyl)-pyrazine and 1-deoxynojirimycin.
Embodiment 2
[0017] Embodiment two, the preparation of extract
[0018] Dried 2000g of Guangdong mulberry leaf fine powder, extracted by diafiltration with 20L 50% ethanol, combined the extracts, recovered until there was no ethanol smell, added an equal volume of ethanol, centrifuged to remove the precipitate, and the supernatant was passed through 732 (H+ type) strong acid ion exchange resin , respectively eluted with 3L of water and 6L of 0.5N ammonia water, and the ammonia water part was concentrated and dried to obtain 5 g of positive ninhydrin reaction containing more than 10% of cytidine, 2-(1', 2', 3', 4'-tetra The total alkaloid extract of hydroxybutyl)-5-(2″, 3″, 4″-trihydroxybutyl)-pyrazine and 1-deoxynojirimycin. The total alkaloid extract was passed through HZ-202 strong Basic ion exchange resin, HD-2 weakly acidic cation exchange resin, Dowex1 * 2 (OH-type) ion exchange resin, Sephadex LH-20 column chromatography separation obtains cytidine (96%, 32mg), 2-(1 ', 2', 3', 4'-te...
Embodiment 3
[0019] Embodiment three, the structure of compound
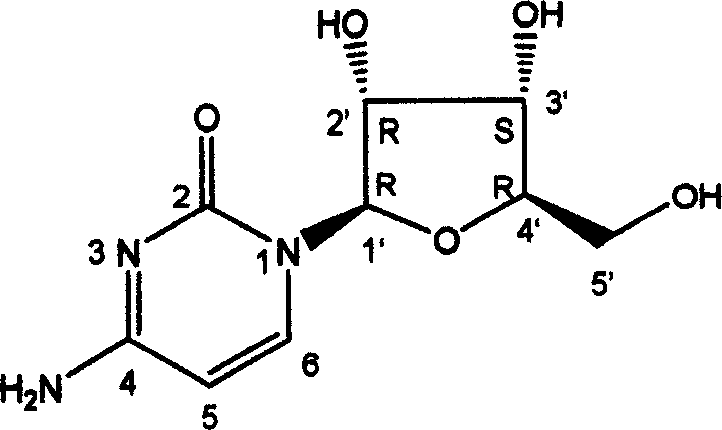
[0020] (1) The structure of cytidine is:
[0021]
[0022] Colorless needle crystal (methanol), C9H13N3O5, ninhydrin is purple. ESI-MS m / z: 266 [M+Na]+, 509 [2M+Na]+. 1H NMR (400MHz, D2O) δ: 7.90 (1H, d, J=7.6Hz, H-6), 6.04 (1H, d, J=7.6Hz, H-5), 5.78 (1H, d, J=3.6 Hz, H-1'), 4.23(1H, t, J=4.8Hz, H-2'), 4.11(1H, t, J=5.6Hz, H-3'), 4.05(1H, m, H- 4'), 3.84 (1H, dd, J=12.8, 2.4Hz, H-5a'), 3.72 (1H, dd, J=12.8, 4.4Hz, H-5b'). 13C NMR (100MHz, D2O) δ: 162.5(C-4), 153.1(C-2), 143.1(C-6), 95.6(C-5), 90.3(C-1'), 84.1(C-4 '), 74.0 (C-2'), 69.2 (C-3'), 60.6 (C-5').
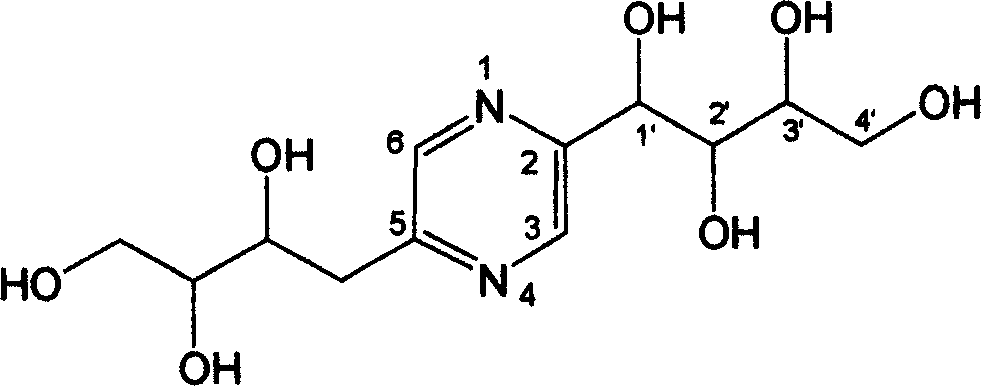
[0023] (2) The structure of 2-(1′, 2′, 3′, 4′-tetrahydroxybutyl)-5-(2″, 3″, 4″-trihydroxybutyl)-pyrazine is:
[0024]
[0025]Colorless needle crystal (methanol), C27H32O14, ninhydrin was purple. ESI-MS m / z: 327 [M+Na]+, 303 [M-H]-. 1H NMR (400MHz, D2O) δ: 8.60 (1H, s, H-3), 8.42 (1H, s, H-6), 5.05 (1H, s, H-1'), 3.94 (1H, m, H -2"), 3.79-3.73(4H, m), 3.60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com