Diazoxide for use in the treatment of a central nervous system (CNS) autoimmune demyelinating disease
A demyelinating disease, the technology of diazoxide, which is applied in the application field of diazoxide in the treatment or prevention of autoimmune demyelinating diseases of the central nervous system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0096] Experiments carried out in mice in the present invention show that diazoxide at most 12mg / m 2 It is effective at different daily doses per day.
[0097] The dose of diazoxide can be expressed as mg diazoxide per kg body weight or mg diazoxide per square meter of roller body surface. The article "Dose translation from animal to human studies revisited" by Reagan-Shaw S. (FASEB J 2007,22:659-661) provides a method for converting mg / kg to mg / m 2 standard conversion factor.
[0098] Dose (mg / kg) x K m = dose (mg / m 2 )
[0099] The article also explains that this conversion is the basis for converting the dose in the first animal species to the dose in the second animal species (dose conversion for body size variation). Thus, a mg / kg animal dose (AD) can be converted to a mg / kg human equivalent dose (HED) by the following formula:
[0100]
[0101] Among them, the Km of each species is shown in Table I (data selected from Reagan-Shaw S. Dose translation from animal ...
Embodiment 1
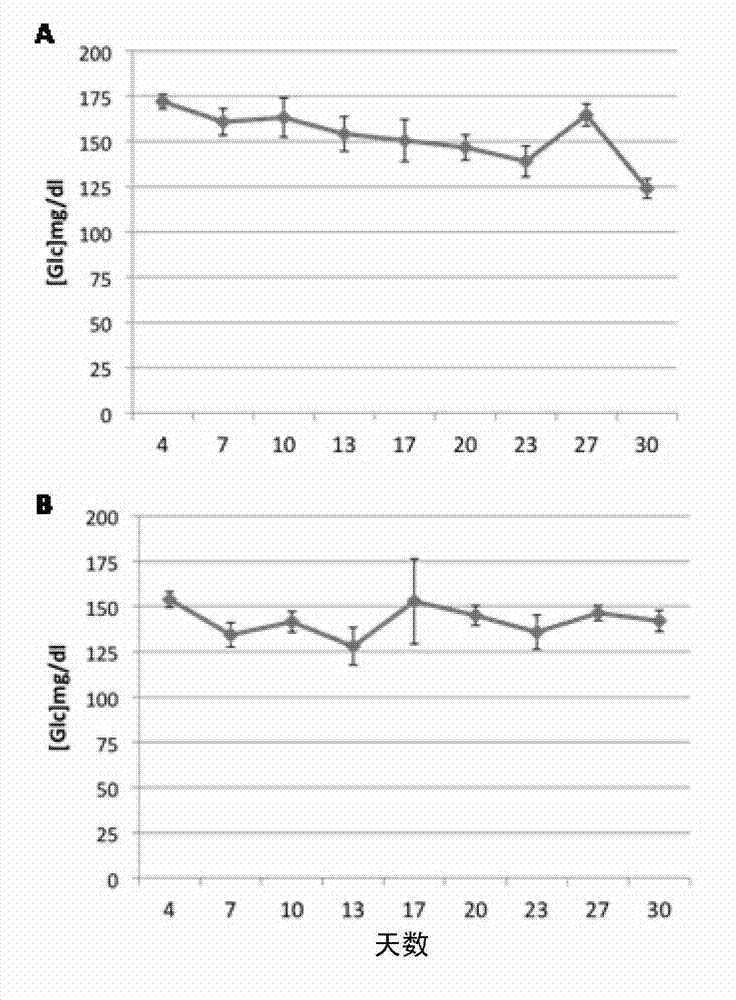
[0107] Example 1: Low doses of diazoxide do not cause hyperglycemia in mice
[0108] To determine the in vivo effect of very low doses of diazoxide on blood glucose, mice receiving daily doses of diazoxide were monitored. Eleven-week-old female C57BL / 6J mice, purchased from Charles River, were maintained on a 12:12-h light:dark cycle with standard food and water ad libitum. Experiment begins at 11:00. Diazoxide (Sigma-Aldrich, St. Louis, Mo, USA) was administered orally by gavage (p.o.) daily at a dose of 1 mg / kg (3 mg / m 2 ) ( figure 1 A) and 0.05mg / kg (0.15mg / m 2 ) ( figure 1 B) (n=6 / group). This period of time is necessary to generate a steady state of plasma diazoxide concentration. Blood glucose levels were measured every 3-4 days during the 30-day treatment period starting on day 4. Measurements were taken immediately before dosing (time 0) and at 60 minutes. Obtain a blood sample from the saphenous vein and measure blood glucose levels using a glucometer and bl...
Embodiment 2
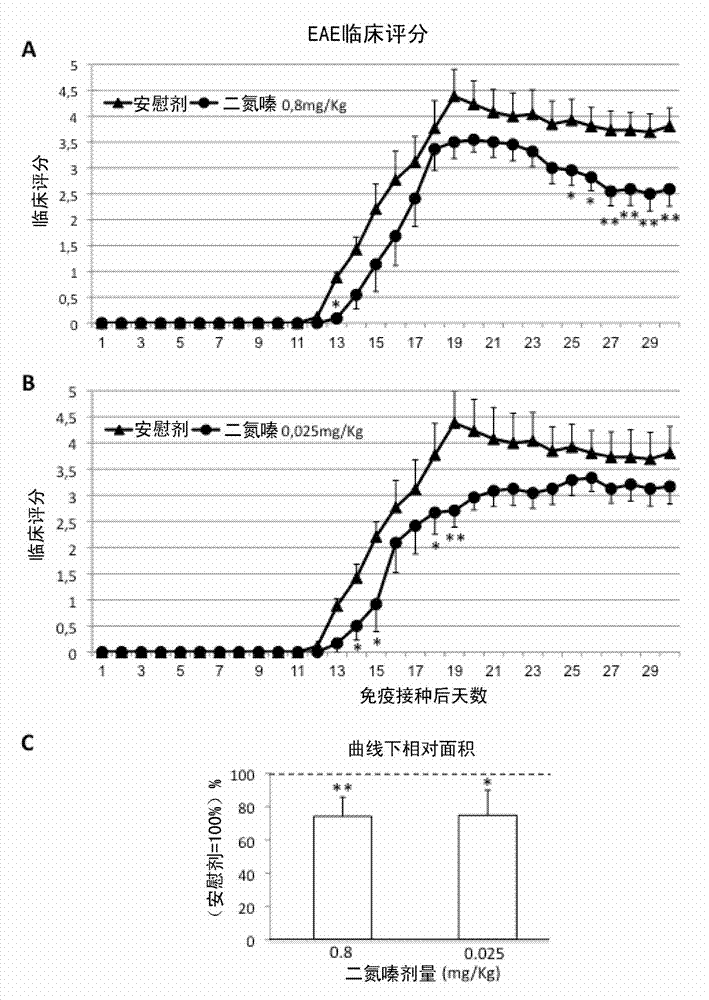
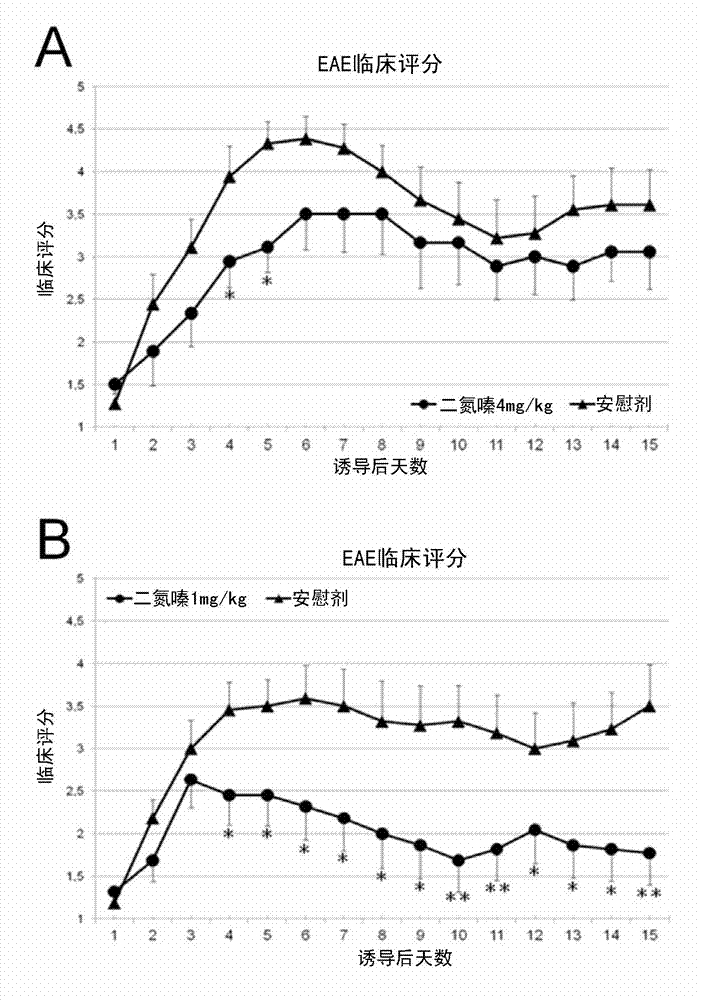
[0109] Example 2: Low-dose diazoxide improves experimental autoimmune encephalomyelitis in mice
[0110] Female C57BL / 6J mice aged 8-10 weeks, purchased from Harlan Laboratories, were maintained on a 12:12 hr light:dark cycle with standard food and water ad libitum. EAE was induced by immunization with >95% pure synthetic myelin oligodendrocyte glycoprotein peptide 35-55 (rat MOG 35-55 , Sequence: MEVGWYRSPFSRVVHLYRNGK; EspiKem Srl, Florence, Italy). Mice were injected subcutaneously on one flank with 100 μl of a solution containing 150 μg of rat MOG (Sigma-Aldrich, St Louis, Mo, USA) in complete Freund's adjuvant and 5 mg / ml of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI, USA). Mice also received one intraperitoneal injection of 150 ng pertussis toxin (Sigma-Aldrich, St. Louis, Mo, USA) in 100 μl PBS
[0111] Mice were scored daily for EAE symptoms on a scale of 0-6, according to the following principles: 0, no clinical symptoms; 1: distal tail weakne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com