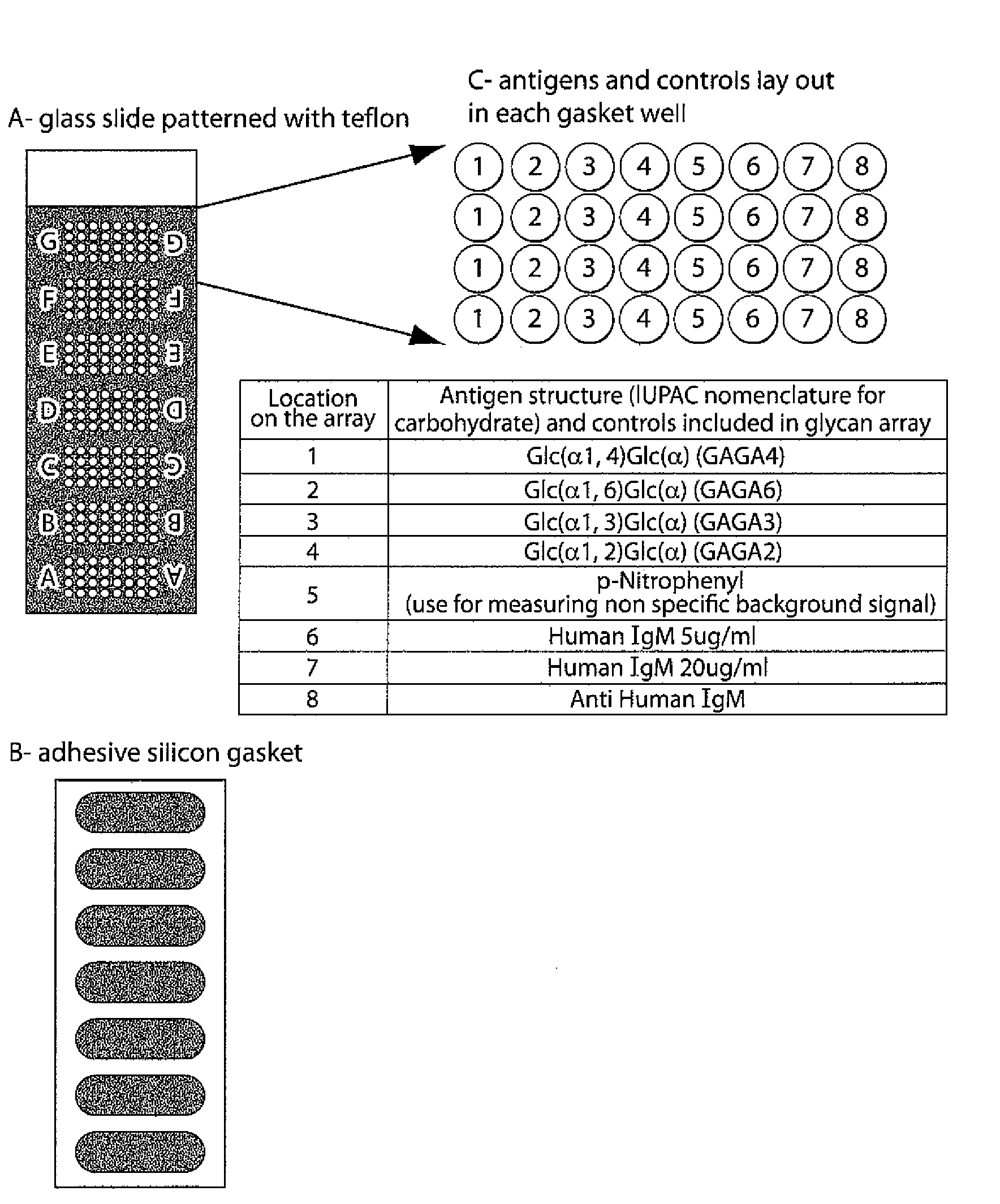
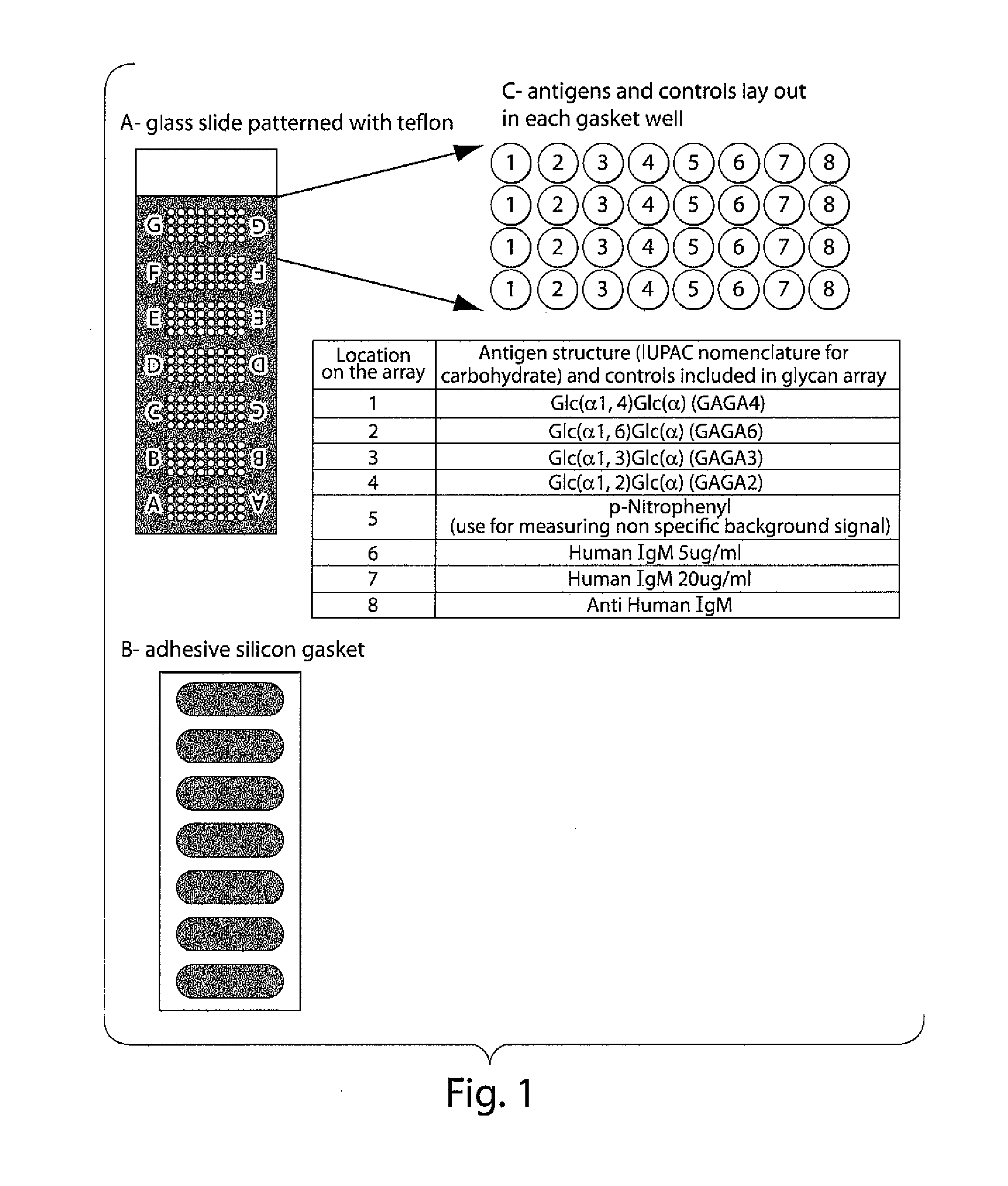
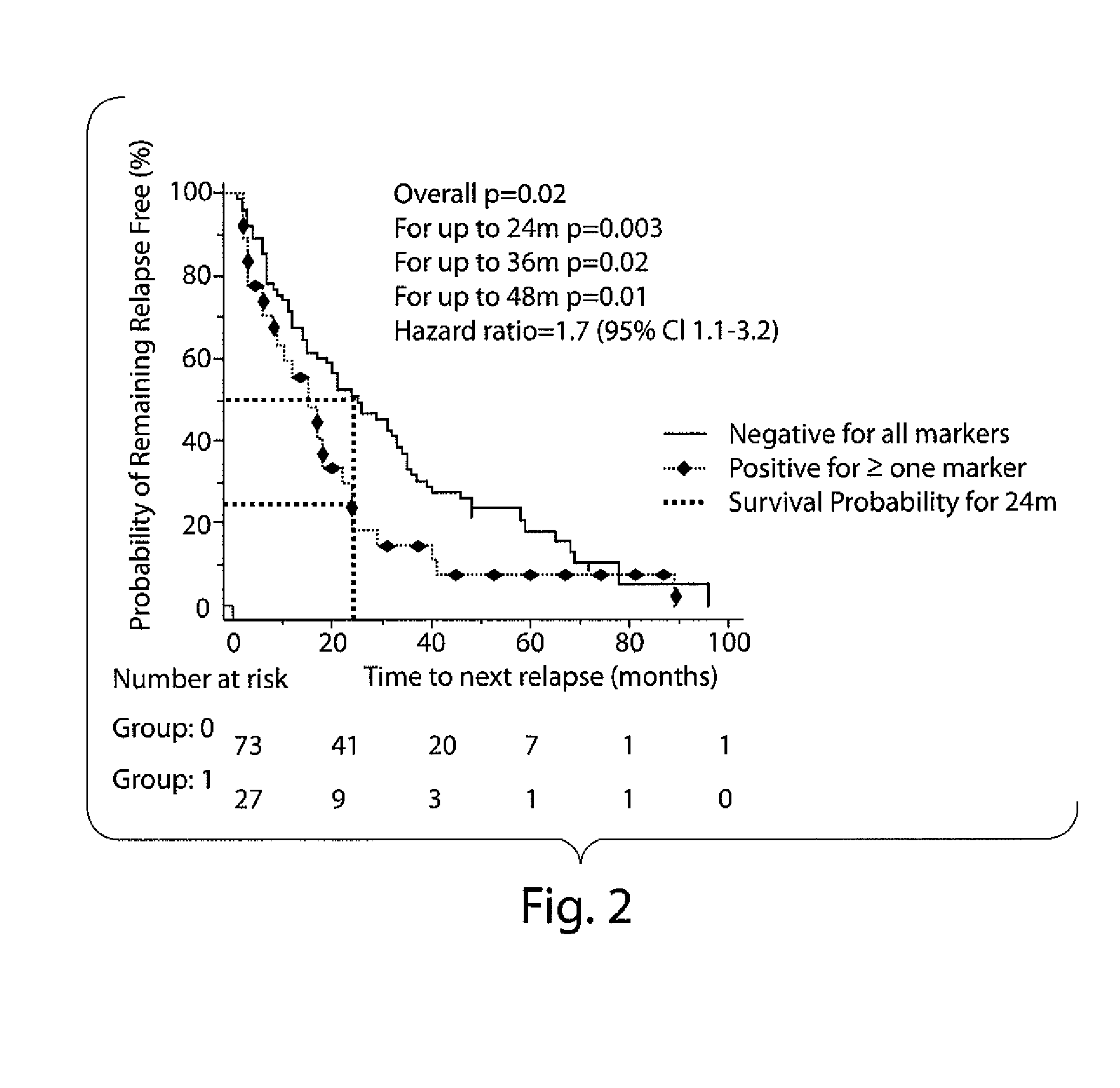
Method for Evaluating Risk in Multiple Sclerosis
a risk assessment and multiple sclerosis technology, applied in the field of multiple sclerosis risk assessment methods and reagents, can solve problems such as persistent disability in young adults
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Anti-α-Glucose Based Glycan IgM Antibodies Predict Relapse Activity in Multiple Sclerosis After the First Presentation
[0051]The results presented herein are from a retrospective study of frozen (−70° C.) and re-thawed serum samples collected from patients at the time of diagnostic work-up for their FP who were later diagnosed as RRMS. The control group included sera samples taken from patients with other neurological diseases (OND) that were stored around the same time from routine samples sent to the respective cerebrospinal fluid (CSF) diagnostic laboratories. Demographic and clinical data were obtained from hospital records. Inclusion criteria for MS samples were as follows: patient age (18-60 years) at time of sampling, follow-up for at least 4 years from blood sampling, and diagnosis of RRMS according to Poser criteria (Poser C M, et al. 1983 Ann Neurol, 13: 227-31). Samples meeting the above criteria were identified from one of two serum repositories located at th...
example 2
Panel of Anti-α-Glucose Based Glycan IgM Antibodies (Anti-GAGA2, Anti-GAGA3, Anti-GAGA4, and Anti-GAGA6) Predict Expanded Disability Status Scale Progression in Clinically Isolated Syndrome Patients Suggestive of Multiple Sclerosis
[0067]Prior to the invention, there was no specific serum based biomarker for the prognosis of Expanded Disability Status Scale (EDSS) progression in clinically isolated syndrome (CIS) patients suggestive of Multiple Sclerosis (MS). For patients with CIS suggestive of MS, there is a need to predict the patients with higher risk for rapid progression in EDSS score. As described in detail below, levels of IgM antibodies to a panel of four glucose-based glycans were analyzed for their ability to predict risk of rapid EDSS progression in CIS patients suggestive of MS within 5 years in patients treated early and those that received delayed treatment.
[0068]A prospective analysis was performed on 286 sera samples taken from CIS patients at baseline in the Betafer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com