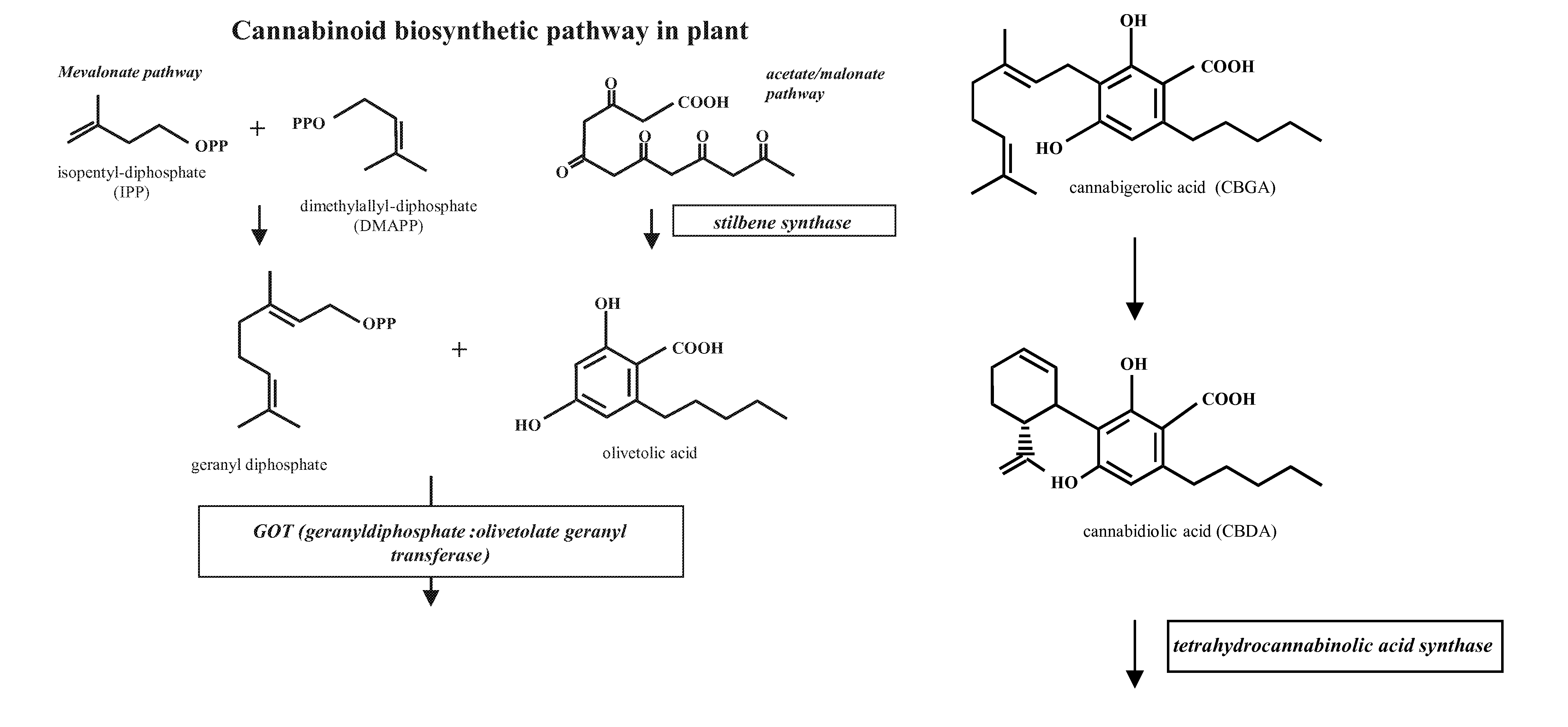
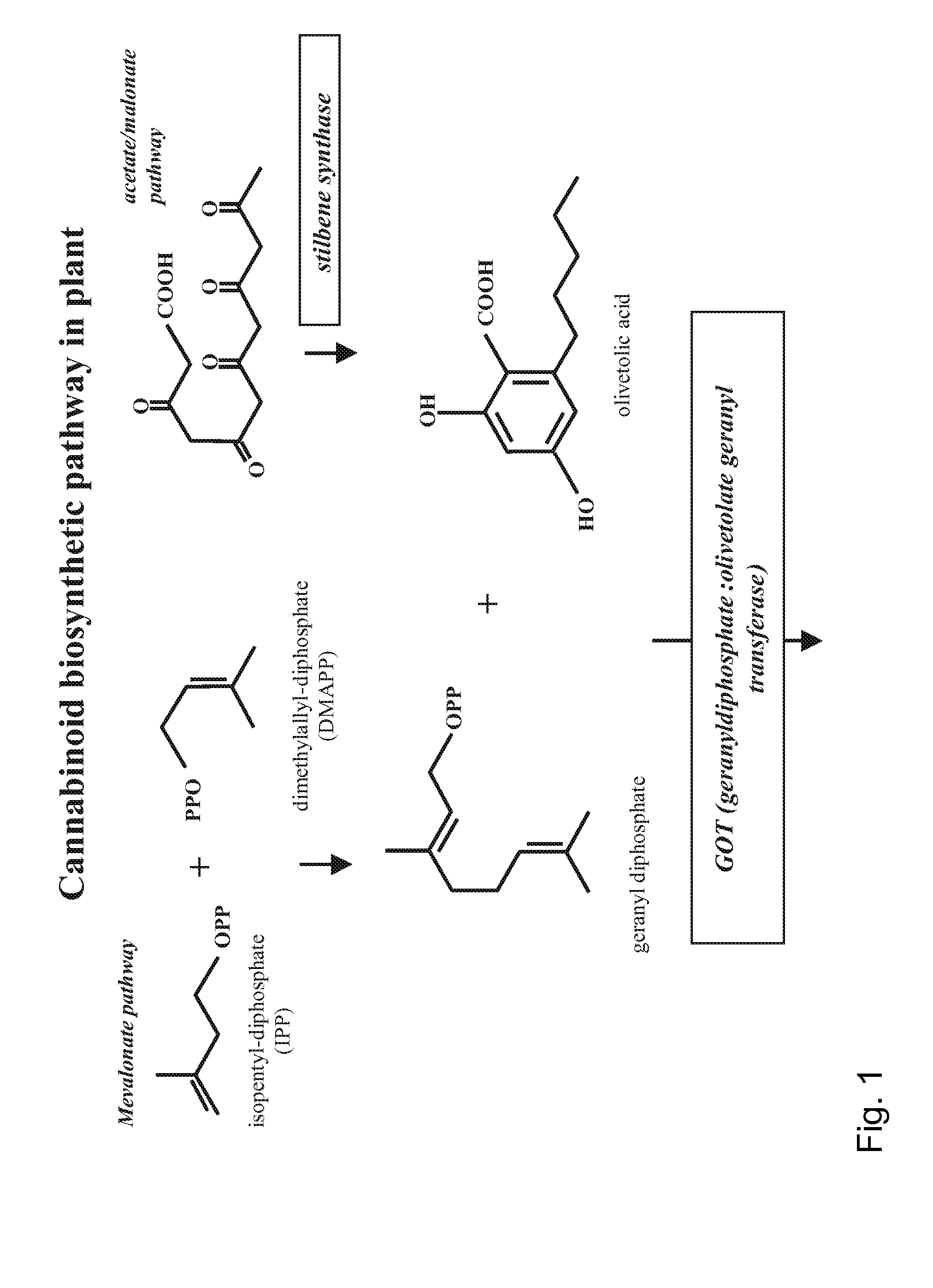
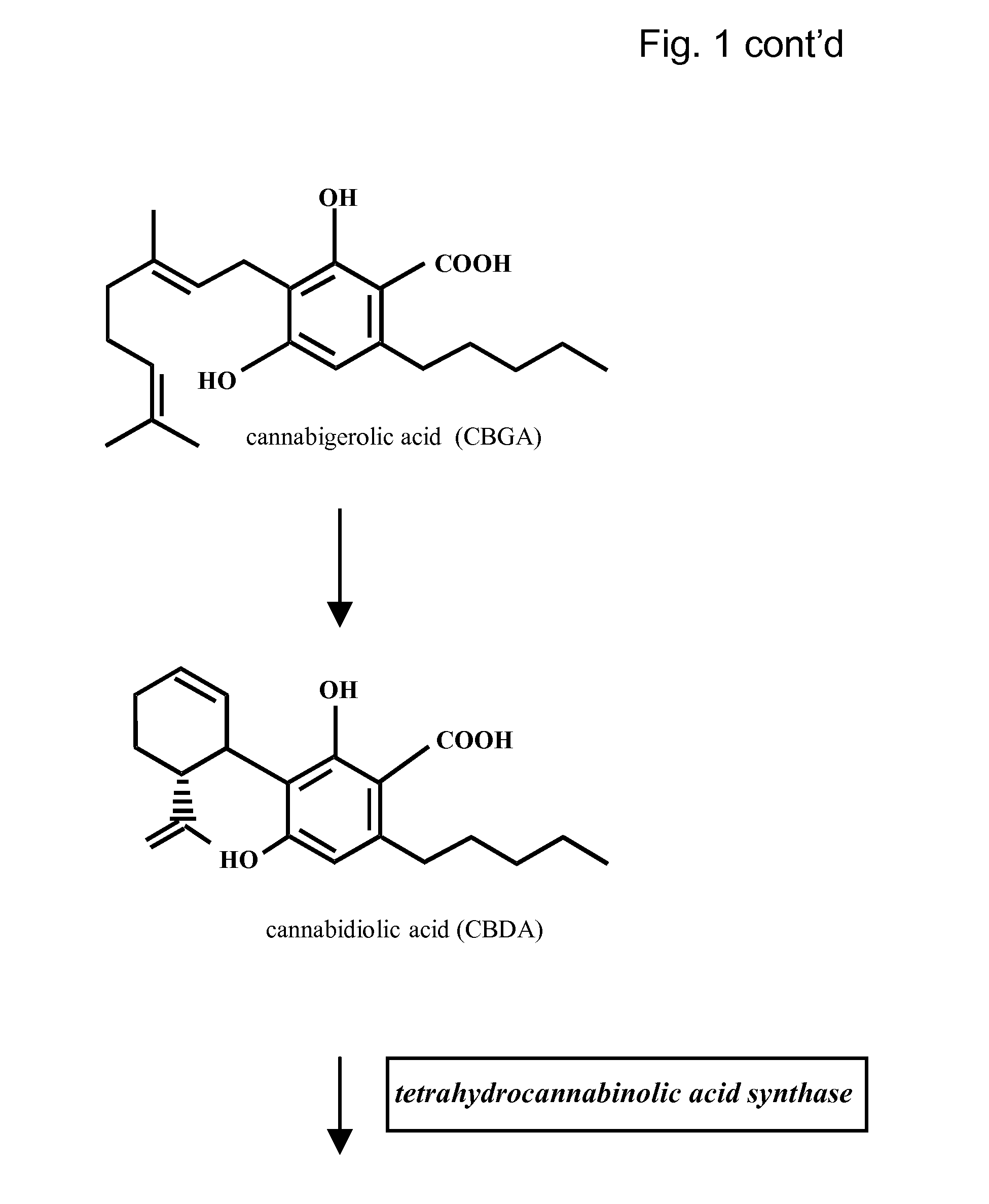
Medicinal Acidic Cannabinoids
a cannabinoid and acidic technology, applied in the field of acidic cannabinoid, can solve problems such as detriment to general health, and achieve the effects of reducing tnf- excretion, increasing interleukin release, and good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Extracts
[0044]Flower tops of three cannabis varieties belonging to C. sativa or C. indica and hybrids. were used to make extracts. The flower tops were deep-frozen immediately after harvesting and thereafter lyophilised, shortly before extraction.
[0045]700 mg dried flower tops were extracted twice with 20 mL chloroform / methanol (1:9), according to the following procedure:[0046]700 mg flower tops were mixed with 18 ml Methanol and sonicated for 5 minutes. 2 mL chloroform were added after which the mixture was sonicated again for 5 minutes. Extraction was then performed (60 minutes 4° C., shaking 250 rpm). Supernatant was removed and the extraction was repeated with the remaining plant-pellet. Both supernatants were pooled and stored at −20° C. until measurements started.
Composition of the Unheated Extracts
[0047]The concentration of THC-A, CBD (the total of cannabidiolic acid and cannabidiol) CBN and THC was determined with LC / MS-MS.
[0048]The results are shown in Ta...
example 2
Receptor Binding Studies
[0049]The affinity of the three extracts for binding to the cannabinoid receptors CB1 and CB2 was determined in a receptor binding study. Herein a competitive assay was used between the components of the extracts and tritium labelled ligand CP55,940. The receptors were recombinant human CB1 and CB2 co-expressed with Gαi131γ proteins in Sf9 cells
[0050]In the binding studies, unheated extracts were compared with extracts heat at 200 C to decarboxylate the THC-A. The affinity constants (Kd) are shown in Table 2.
TABLE 2ExtractKd CB1 [μM]Kd CB2 [μM]Cultivar 1 unheated>1>1Cultivar 2 unheated>1>1Cultivar 3 unheated>1>1Cultivar 1 heated0.00620.019Cultivar 2 heated0.00790.021Cultivar 3 heated0.0170.023
[0051]A compound with a low Kd is generally considered as a potential anti-inflammatory agent or as a potential analgesic. From the much higher Kd values from the unheated (undecarboxylated) extract, one would expect that the acidic cannabinoids would not be promising ag...
example 3
Biological Immuno-System Based Assay
[0055]U937 monocytes (described e.g. in Izeboud et al., J. Rec. Sign. Tr. Research (1999), 19(1-4):191-202) were differentiated into macrophages by treating the monocytes for 16 hours with phorbol myristate acetate (PMA)
[0056]After 48 hours storage of the macrophages in RPMI-1640 culture medium wherein the medium was replaced every 24 hours. The macrophages were allowed to recover from PMA treatment for 48 hours, during which culture medium was replaced every 24 hours. At day three after PMA treatment, the macrophages were exposed to lipopolysacharide (LPS) (Sigma-Aldrich, L-2630) The macrophages were exposed to LPS in the presence or absence of the cannabis extracts described above (in methanol). The extracts were tested undiluted and in 2.5-fold, 5-fold, 7.5-fold and 10-fold dilution). In the culture medium the TNF-α level was determined a by specific ELISA test (TNFα Cytoset, Biosource CHC1754). Further, the toxicity of the cannabis extracts wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com