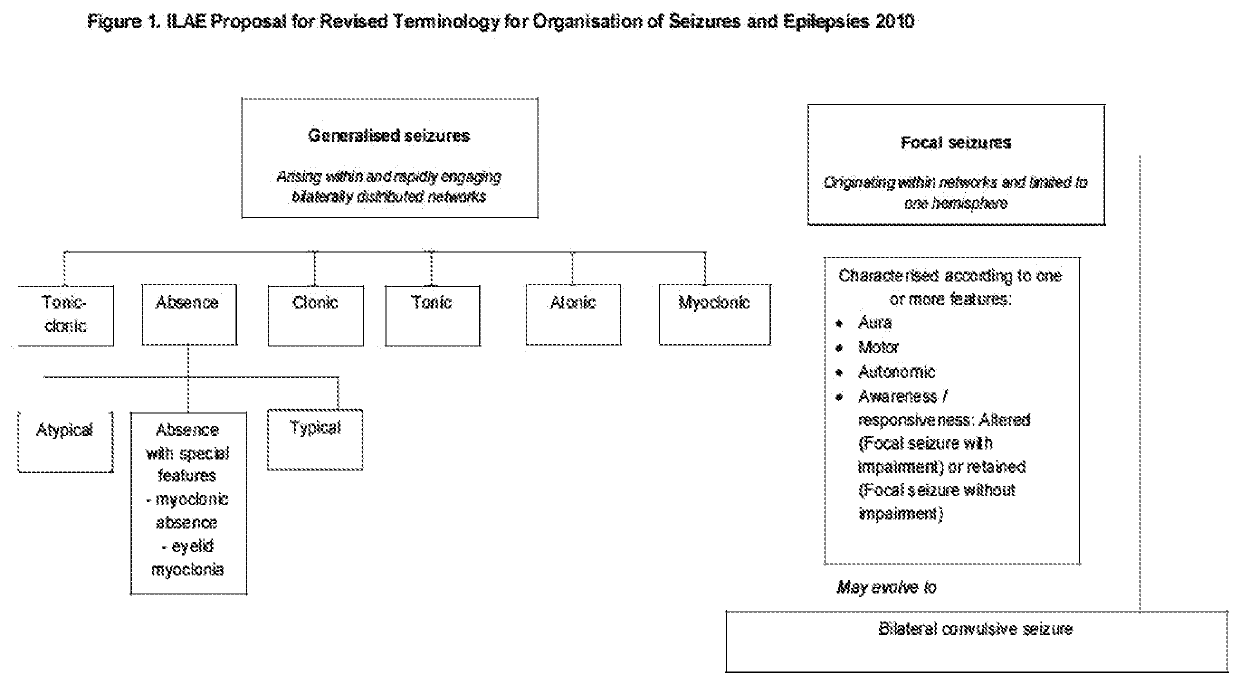
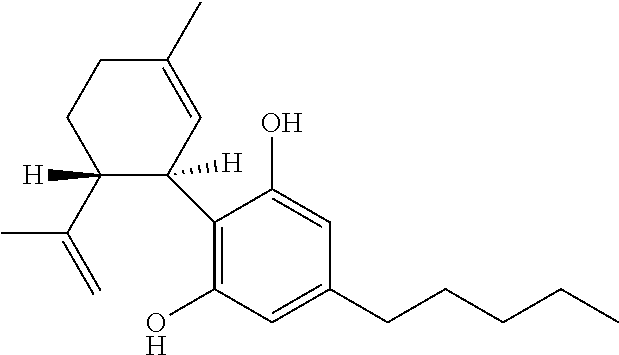
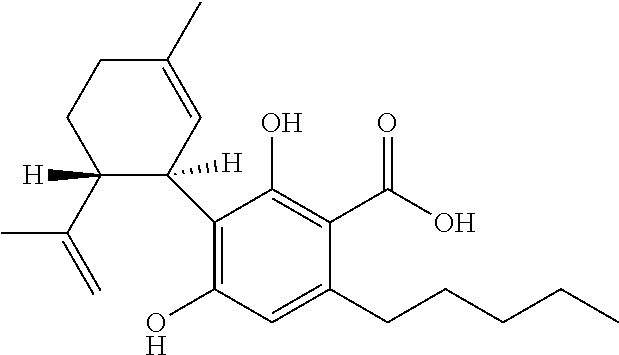
Use of cannabinoids in the treatment of epilepsy
a cannabinoid and epilepsy technology, applied in the field of epilepsy treatment with cannabinoids, can solve the problems of difficult treatment, inability to obtain seizure freedom, cognitive, behavioral and motor delays, etc., to reduce the number of seizures, reduce the number of different anti-epileptic drugs that are used in combination with cbd, and achieve safe side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Cannabidiol in Reducing Seizures and Other Symptoms in Children and Young Adults with Sturge Weber Syndrome
Materials and Methods
[0094]Four subjects with SWS brain involvement and refractory epilepsy were enrolled in an expanded access compassionate use program for CBD. These subjects were treated with a highly purified extract of cannabidiol (CBD) obtained from a cannabis plant. Frequency of seizures was recorded at each visit, as were reported quality of life changes, including mood, behaviour, and cognitive function.
[0095]Data were compared in the 56-day pre-treatment period, the 56-day period after starting maintenance dose (Week 14), and at most recent visit.
[0096]The participants in the study were taking at the time of entry into the study between one and four concomitant AEDs.
[0097]Patient 1 was taking leviteracetam alone. Patient 2 was taking leviteracetam, valproate, felbamate and clobazam. Patient 3 was taking valproate and topiramate. Patient 4 was taking oxycarbamazepi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com