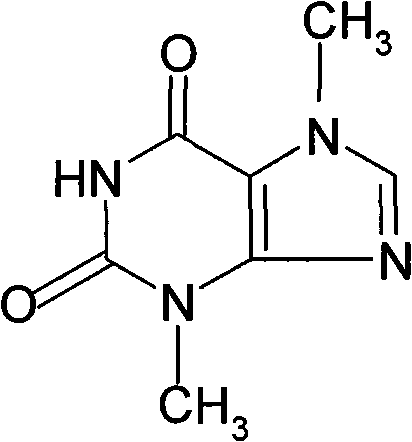
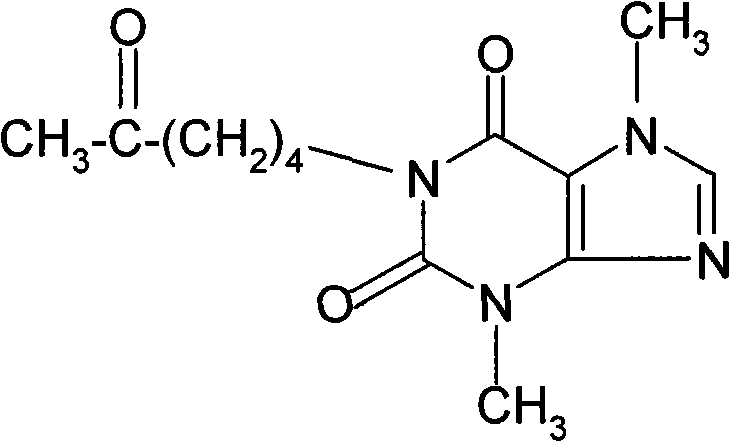
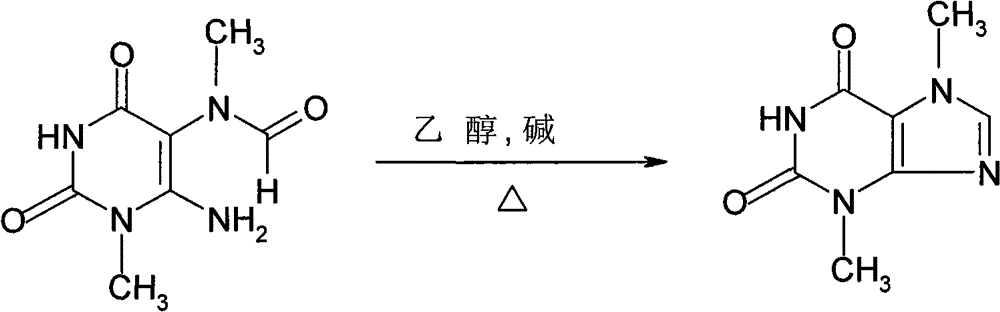
Theobromine production process
A technology of theobromine and solvent, applied in the field of preparation and refining of theobromine, can solve the problems of flammability, difficulty in removal, toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0086] This example demonstrates the preparation of theobromine in a mixture of acetone and water in the presence of sodium bicarbonate.
[0087] 16.6 g of 3-methylxanthine (0.1 mol) and 100 ml of acetone were mixed in a 500 ml four-necked reaction vessel equipped with a mechanical stirrer, and 20 ml of water were added. 36 g of sodium bicarbonate were added with stirring and heated to a temperature of 55-60°C. 20 g of dimethyl sulfate were added dropwise over 3.5 hours and kept stirring at 55-60° C. for half an hour. Then, acetone is distilled off and recovered. 50 ml of water were added and the reaction vessel was heated to 80°C. Then, 32% hydrochloric acid was slowly added to the mixture, and the measured pH was in the range of 5-6. The mixture was cooled to 25°C, whereby the solid formed was obtained by precipitation, washed with water and dried at 70°C; 15.7 g of theobromine were obtained in 87% yield with a purity of 99.9% by HPLC.
example 3
[0089] This example demonstrates the preparation of theobromine in the presence of sodium carbonate in acetone.
[0090] In a 500ml four-necked reaction vessel equipped with a stirrer, 3-methylxanthine disodium salt (63g), acetone (304ml) and sodium carbonate (19.1g) were added with continuous stirring. The mixture was slowly heated to about 55°C. Dimethyl sulfate (60 g) was added dropwise over 4 hours. Then, the reaction was kept at 55°C for half an hour. Acetone was distilled off at normal pressure and water (50ml) was added. The product solution was neutralized to a pH of around 5 using 32% hydrochloric acid at 30°C. Then, the solution was filtered, washed with warm water (15 ml) at 40°C, filtered under reduced pressure to remove water, and dried at 70°C to obtain crude theobromine in 86.7% yield.
example 4
[0092] This example demonstrates the preparation of theobromine in acetone in the presence of sodium carbonate.
[0093] In a 500ml four-necked reaction vessel equipped with a stirrer, 3-methylxanthine disodium salt (63g), acetone (304ml) and sodium carbonate (19.1g) were added under stirring. The mixture was heated to 55°C and dimethyl sulfate (60 g) was added dropwise over 3.5 hours. Then, the reaction was maintained at 55°C for another half hour. Acetone was evaporated at atmospheric pressure. Water (50ml) was added. The resulting product solution was neutralized to a pH of around 6 using 32% industrial hydrochloric acid at 30°C. Then, the solution was filtered, washed with warm water (10 ml) at 40°C, filtered under reduced pressure to remove water, and dried at 70°C to obtain theobromine in 84.3% yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com