Preparation method for Trajenta
A compound and preset temperature technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, no large-scale production, high cost, etc., and achieve the effect of meeting the needs of industrial production, low production cost, and avoiding dimer impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
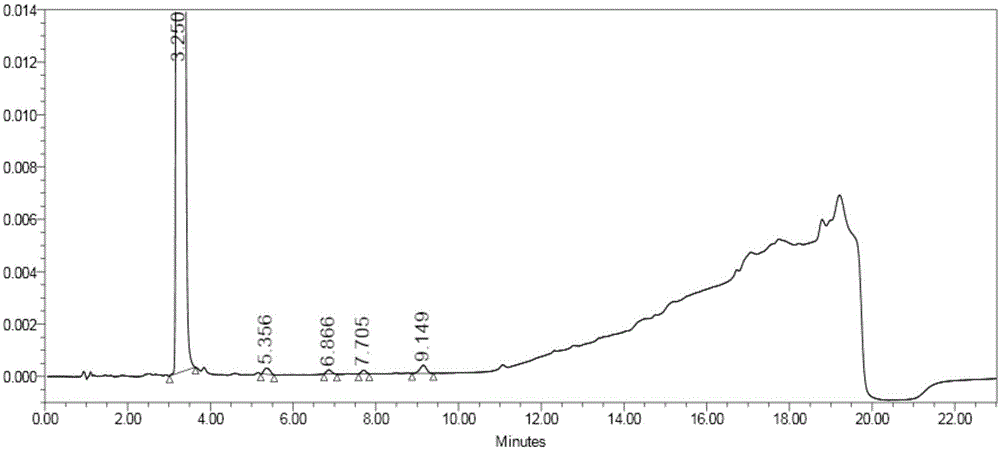
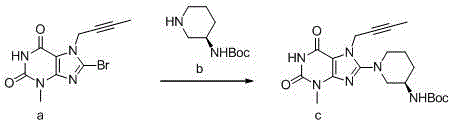
[0049] Step 1: Add 10.0g of compound a, 8.0g of compound b, and 7.0g of potassium carbonate into 100mL of N-methylpyrrolidone in turn, stir and heat up to 120°C for reaction, monitor by TLC, after the reaction is complete, cool down to 20°C, and dissolve the reaction solution Poured into 500mL of purified water, precipitated solid, filtered, and air-dried at 45°C to obtain compound c with a mass of 11.5g;
[0050] Step two:
[0051]
[0052] Add 5.0g of compound c to 25mL of dichloromethane, add 15mL of trifluoroacetic acid pretreated by distillation, react at 20°C, and monitor by TLC. After the reaction is complete, add 100mL of dichloromethane and 200mL of water to the reaction solution in sequence, Stir for 1 hour, separate the layers, wash the water layer once with 50 mL of dichloromethane, separate the layers, add 100 mL of dichloromethane again to the water layer, adjust the pH to 10-11 with 20% by mass sodium hydroxide solution, and separate the layers ,...
Embodiment 2
[0059]
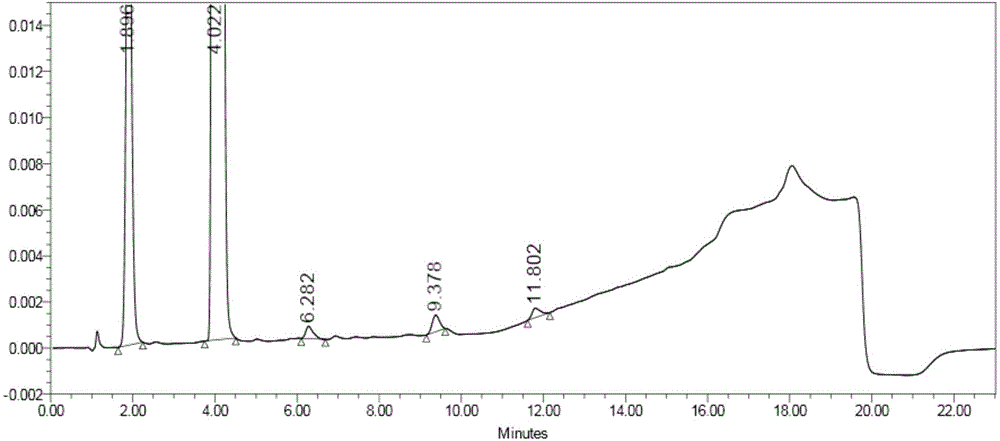
[0060] Step 1: Add 2.0g of compound a, 2.0g of compound b, and 1.4g of potassium carbonate into 20mL of N,N-dimethylformamide in turn, stir and heat up to about 110°C for reaction, monitor by TLC, after the reaction is complete, cool down to 30°C ℃, pour the reaction liquid into 100mL of purified water, precipitate solid, filter, and blow dry at 45℃ to obtain compound c with a mass of 2.1g;
[0061] Step two:
[0062]
[0063] Add 5.0g of compound c to 25mL of dichloromethane, add 15mL of trifluoroacetic acid / dichloromethane solution pretreated by distillation, react at 10°C, monitor by TLC, after the reaction is complete, add 100mL of dichloromethane to the reaction solution in turn and 200mL of water, stirred at room temperature for 1h, separated, the water layer was washed once with 50mL of dichloromethane, separated, and 100mL of dichloromethane was added to the water layer again, and the pH was adjusted to 10 with 20% sodium hydroxide solution. -11, liquid...
Embodiment 3
[0070]
[0071] Step 1: Add 2.0g of compound a, 2.7g of compound b, and 1.4g of potassium carbonate to 20mL N,N-dimethylformamide in turn, stir and heat up to about 115°C for reaction, monitor by TLC, after the reaction is complete, cool down to 25°C °C, pour the reaction solution into 100 mL of purified water, precipitate solid, filter, and blow dry at 45 °C to obtain compound c with a mass of 1.8 g;
[0072] Step two:
[0073]
[0074] Add 5.0g of compound c to 25mL of dichloromethane, add 15mL of trifluoroacetic acid / dichloromethane solution pretreated by distillation, react at 30°C, monitor by TLC, after the reaction is complete, add 100mL of dichloromethane to the reaction solution in turn and 200mL of water, stirred at room temperature for 1h, separated, the water layer was washed once with 50mL of dichloromethane, separated, and 100mL of dichloromethane was added to the water layer again, and the pH was adjusted to 10 with 20% sodium hydroxide solution. -11, liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com