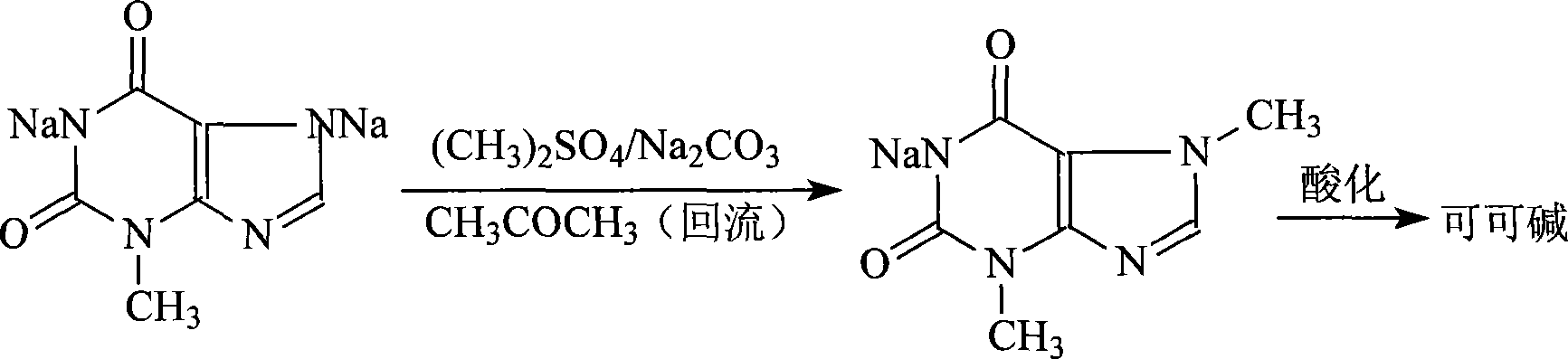Novel method for producing theobromine with 3_methylxanthine disodium salt methylating
A technology of methylxanthine disodium salt and theobromine, applied in the direction of organic chemistry and the like, can solve the problems of incomplete reaction of raw materials, low yield, and high by-product caffeine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] In a 500ml four-necked reaction flask with stirring, add 63 grams of 3-methylxanthine disodium salt, 304ml of acetone, and 19.1 grams of sodium carbonate, slowly raise the temperature to a slight reflux of 55°C while stirring, and add dimethyl sulfate dropwise Add 60 grams in 3.5 hours, then continue the reaction at 55°C for 0.5 hours, distill off the acetone under normal pressure, add 50ml of water, neutralize with 32% industrial hydrochloric acid at 30°C until the pH reaches 6, filter, wash with 10ml of warm water at 40°C, Drained and dried at 70°C to obtain the crude product of theobromine with a yield of 84.3%.
Embodiment 2
[0013] In a 1000ml four-necked reaction flask with stirring, add 126 grams of 3-methylxanthine disodium salt, 610ml of acetone, and 38.5 grams of sodium carbonate, slowly raise the temperature to a slight reflux of 55°C while stirring, and add dimethyl sulfate dropwise Add 120 grams in 3.5 hours, then continue the reaction at 57°C for 0.5 hours, distill off the acetone under normal pressure, add 100ml of water, neutralize with 32% hydrochloric acid at 30°C until the pH reaches 6, filter, wash with 20ml of warm water at 40°C, and pump Dry at 70°C to obtain the crude product of theobromine with a yield of 84.9%.
Embodiment 3
[0015] In a 500ml four-necked reaction flask with stirring, add 63 grams of 3-methylxanthine disodium salt, 304ml of acetone, and 31 grams of sodium carbonate, slowly raise the temperature to a slight reflux of 55°C while stirring, and dropwise add dimethyl sulfate 60 gram, after adding in 4 hours, continue to react at 60°C for 1 hour, distill off acetone under normal pressure, add 40ml of water, neutralize with 32% hydrochloric acid at 30°C until pH reaches 5, filter, wash with 15ml of warm water at 40°C, and drain The crude product of theobromine was dried at 70°C with a yield of 86.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com