Pharmaceutical composition for the prevention or the treatment of non-alcoholic fatty liver disease and the method for prevention or treatment of non-alcoholic fatty liver disease using the same
A fatty liver disease, non-alcoholic technology, applied in the field of pharmaceutical composition for preventing or treating non-alcoholic fatty liver disease and using the pharmaceutical composition to prevent or treat non-alcoholic fatty liver disease, can solve the problem of C To prevent and treat liver fibrosis or cirrhosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the tartrate of compound 1 and sitagliptin phosphate are to high-fat feed-induced Preventive effect of simple steatosis in mice
[0071] In order to investigate the preventive effect of the tartrate salt of Compound 1 (prepared according to the method described in Korean Patent Application No. 2008-0036052) and sitagliptin phosphate on simple steatosis, the following experiments were performed.
[0072] 6-week-old male C57BL / 6 mice were stabilized and then divided into 5 groups. One group was given a standard feed containing 10% fat (trade name: D 12450B, produced by Research Diets), and one group was given a high-fat feed containing 60% fat (trade name: D12492, produced by Research Diets). The remaining three groups were drug-treated groups given a specially formulated drug-mixed feed by mixing a high-fat feed with the drug. For the tartrate salt of compound 1, in order to provide 100 mg / kg and 300 mg / kg (the daily target dose of the tartrate salt of...
Embodiment 2
[0078] Embodiment 2: The tartrate of compound 1 is to the rat simple steatosis induced by high-fat diet sexual healing
[0079] In order to investigate the effect of the tartrate salt of Compound 1 (prepared according to the method described in Korean Patent Application No. 2008-0036052) on simple steatosis, the following experiments were performed.
[0080] 6-week-old male rats (Wistar rats) were stabilized and then divided into 2 groups. The animal groups were given a standard feed containing 10% fat (trade name: D 12450B, produced by Research Diets) and a high-fat feed containing 60% fat (trade name: D12492, produced by Research Diets) for 24 weeks. When the feed consumption was calculated at the 22nd week when the feed was provided, the high-fat feed fed group showed a feed intake of 33.40 g / kg. In order to provide a daily target dose of 10 mg / kg of the tartrate salt of Compound 1, the feed was formulated by mixing a high fat feed with 0.03% by weight of the tartrate ...
Embodiment 3
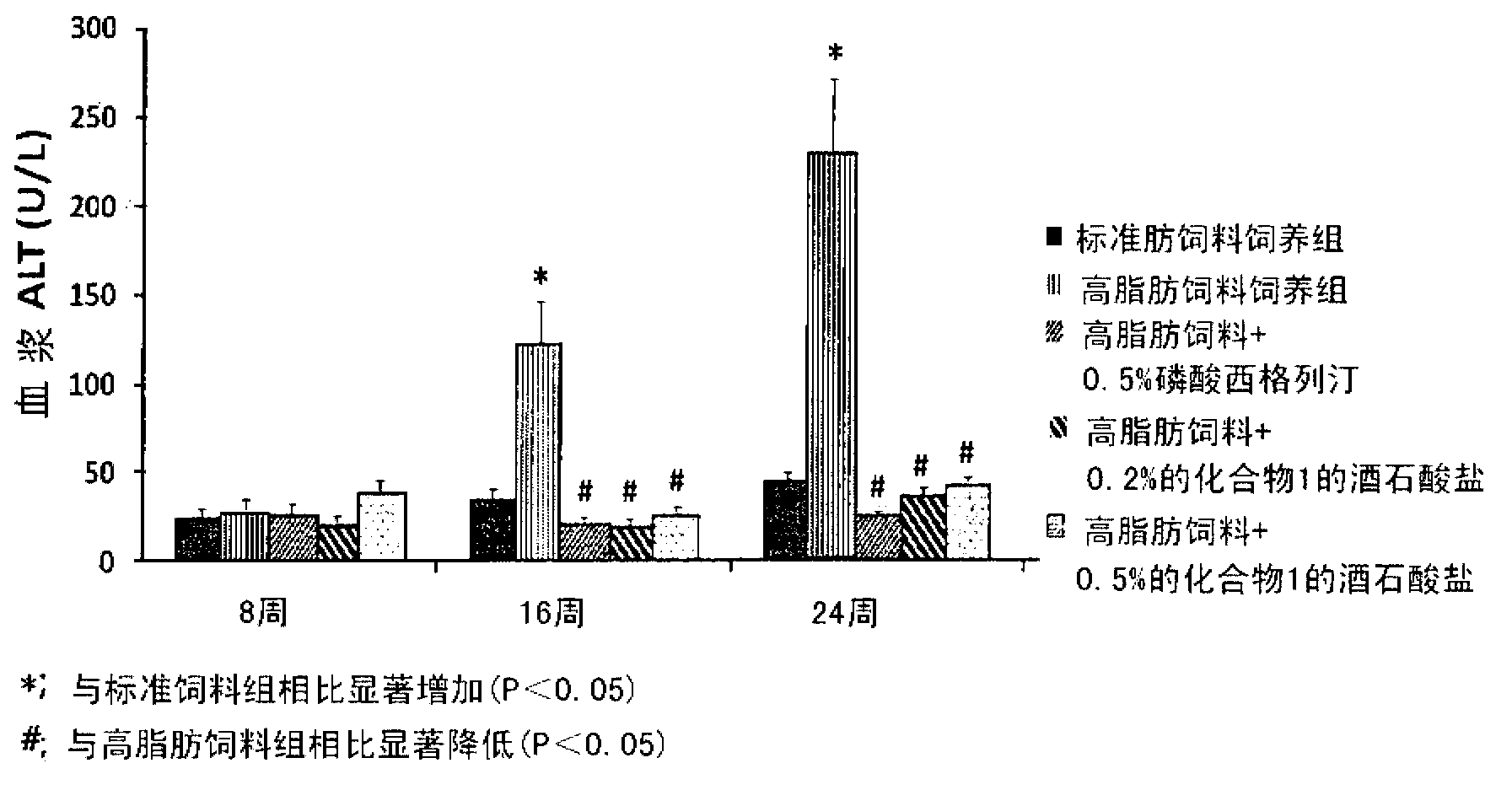
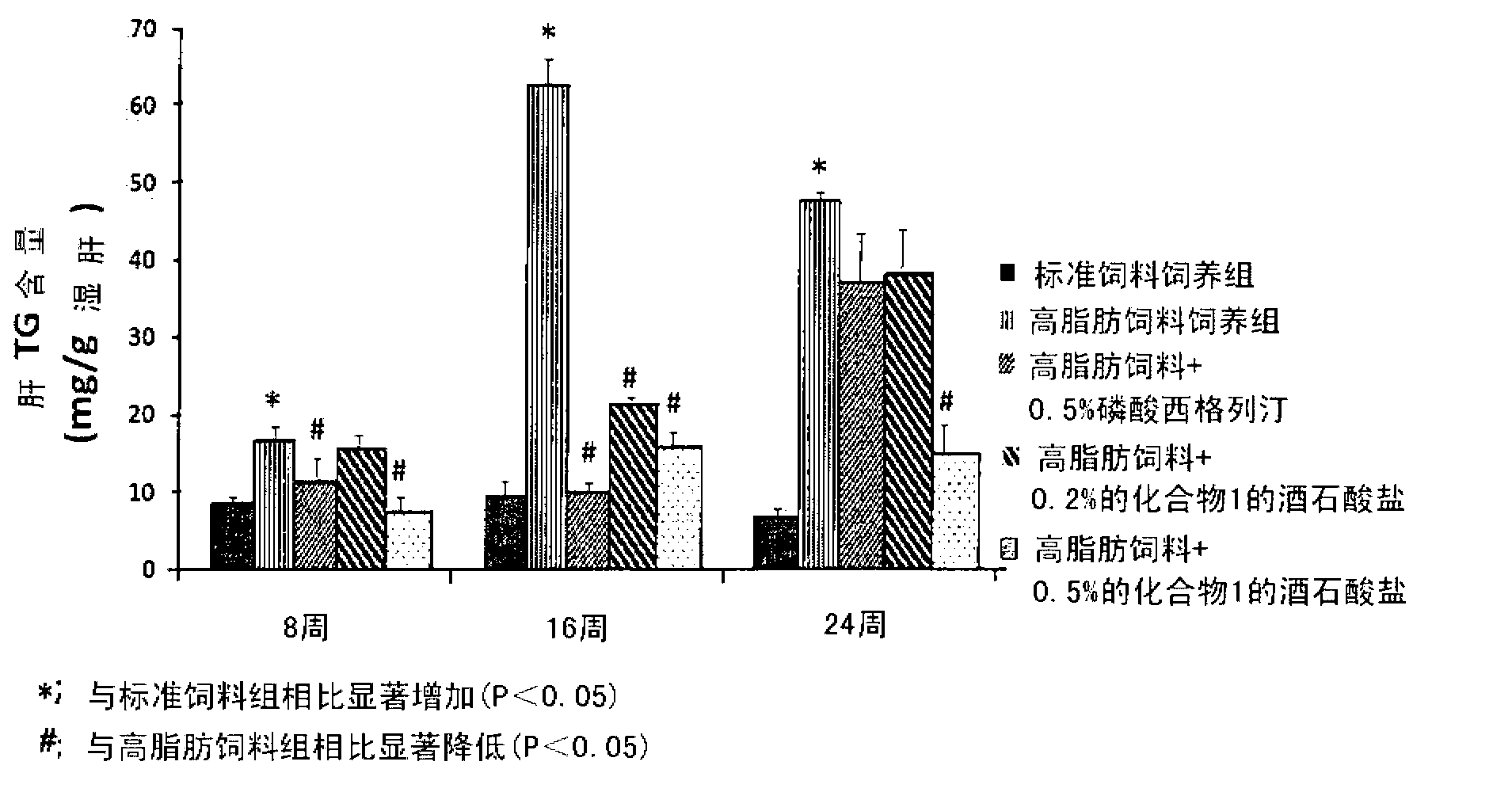
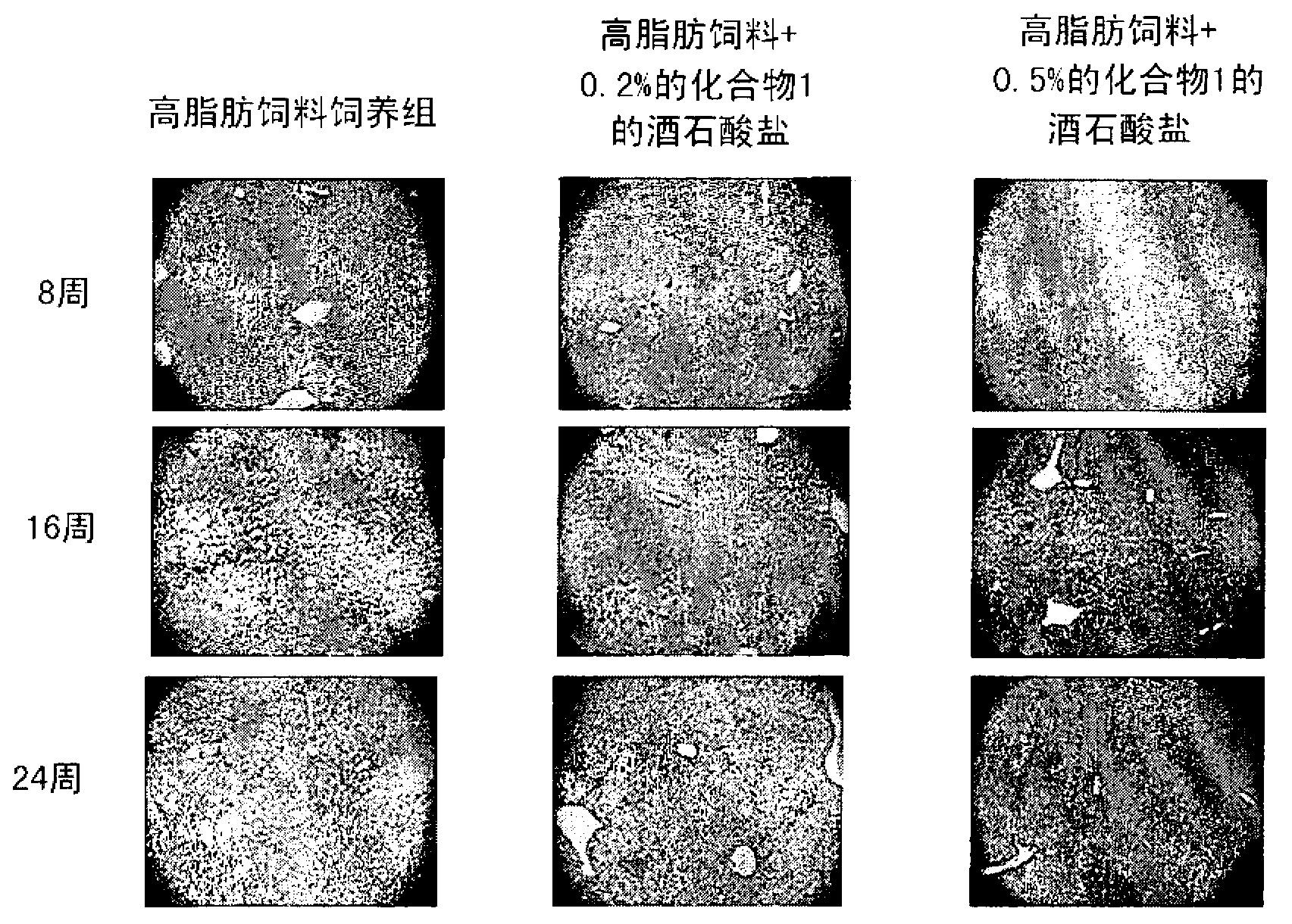
[0082] Embodiment 3: the tartrate of compound 1, sitagliptin phosphate and vildagliptin are to high fat Diet-induced simple steatosis prevents non-alcoholic fatty liver disease in mice
[0083] In order to investigate the preventive effect of the tartrate salt of Compound 1 on non-alcoholic fatty liver (simple steatosis), the following experiments were performed.
[0084] 7-week-old male C57BL / 6 mice were stabilized and then divided into nine groups (n = 9) based on body weight and blood glucose levels. A vehicle solution (0.5% MC (methylcellulose)) of each feed was orally administered once a day to the standard feed-fed group and the high-fat feed-fed group at a dose of 10 ml / kg, and corresponding to the named groups, To the rest of the group orally administered once a day with the tartrate of Compound 1 at doses of 30, 100 and 300 mg / kg, sitagliptin phosphate at doses of 100 and 300 mg / kg, and vitamin C at doses of 100 mg / kg and 300 mg / kg, respectively. Gliptin mixed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com