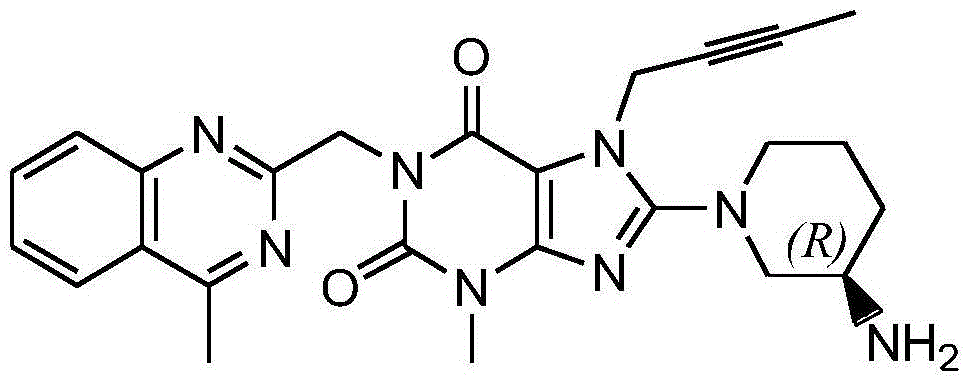
Preparation method for linagliptin
A technology of methyl and purine, which is applied in the field of preparation of raw materials, can solve the problems of restricting industrialized large-scale production, increasing production costs, and difficulty in feeding and proportioning, and achieves the effects of shortening reaction time, improving purity, and reducing reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
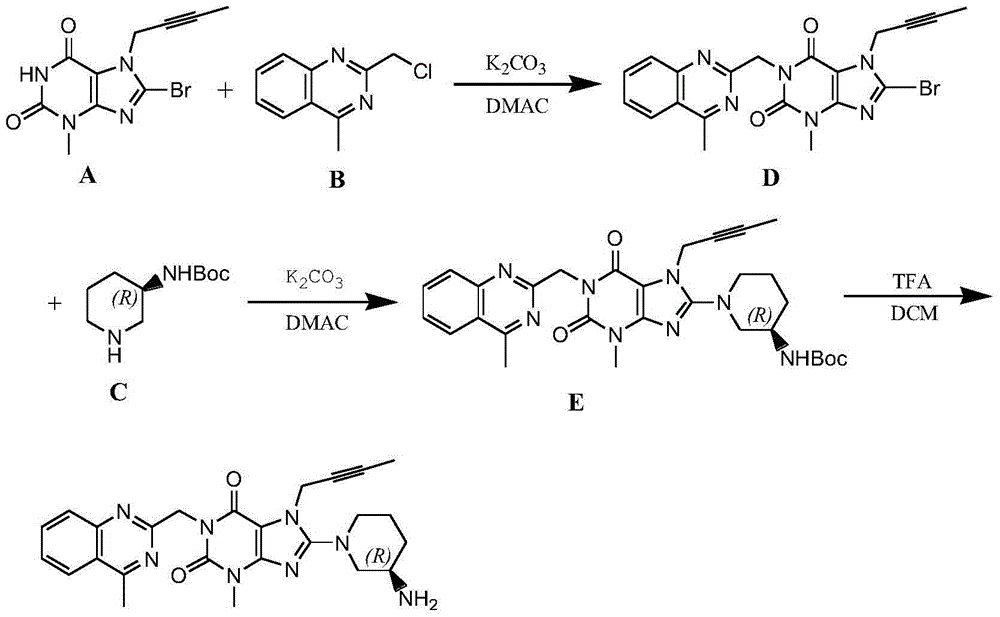
[0050] Compound A (8-bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1H-purine-2,6-dione) (10 g, 33.7 mmol) was added to compound N-methyl- 2-Pyrrolidone 30ml solution, reacted at 55~60°C for 4 hours, then added 50 μm sodium carbonate (5.4g, 50.55mmol), and added compound C ((R)-3-tert-butoxycarbonylaminopiperidine) ( 8.1g, 40.4mol), reacted at 55-60°C for 6h, added 60ml of pure water after the reaction, precipitated solid, filtered, dissolved the wet filter cake with 80ml of dichloromethane, washed with water, dried with anhydrous sodium sulfate, filtered, Concentrate under reduced pressure to 20ml, add 80ml of n-hexane, precipitate a solid, filter, and dry to obtain the linagliptin intermediate E (1-[(4-methyl-quinazolin-2-yl)methyl]-3- Methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-yl]-2,6-dione-2, 3,6,7-tetrahydro--1H-purine) 17.7g, the yield was 91.8%, and the purity was 99.6%.
[0051] Dissolve intermediate E (15g, 26.2mmol) in 150ml of dichlorometha...
Embodiment 2
[0053] Add compound A (10g, 33.7mmol) to compound B (7.8g, 40.4mmol) and 100μm sodium carbonate (5.4g, 50.55mmol) in N-methyl-2-pyrrolidone 30ml solution, at 55~60°C React for 4 hours, then add 100 μm sodium carbonate (5.4g, 50.55mmol), and add compound C (8.1g, 40.4mol) at the same time, react at 55-60°C for 6h, add 60ml of pure water after the reaction, precipitate solid, filter , the wet filter cake was dissolved in 80ml of dichloromethane, washed with water, dried with anhydrous sodium sulfate, filtered, concentrated to 20ml under reduced pressure, added 80ml of n-hexane, precipitated solid, filtered, and dried to obtain Linagliptin intermediate E17.6g , the yield was 91.3%, and the purity was 99.5%.
[0054] Dissolve intermediate E (15g, 26.2mmol) in 150ml of dichloromethane, cool to below 10°C, add 37.5ml of trifluoroacetic acid dropwise, after the dropwise completion, react at room temperature for 5 hours, add dropwise an aqueous potassium carbonate solution with a mass...
Embodiment 3
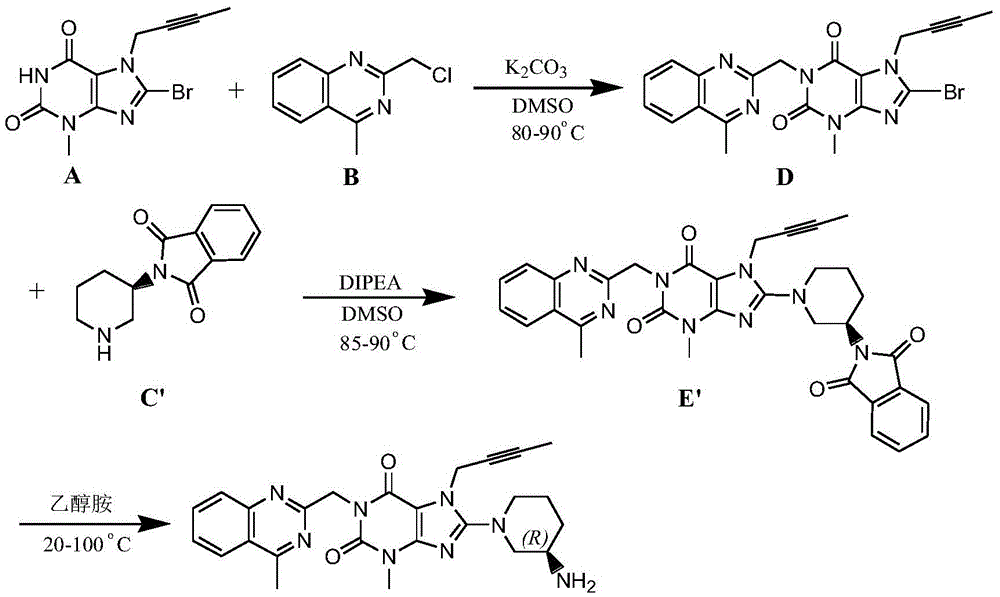
[0056] Add compound A (10g, 33.7mmol) to compound B (7.8g, 40.4mmol) and 200μm sodium carbonate (5.4g, 50.55mmol) in N-methyl-2-pyrrolidone 30ml solution, at 55~60°C React for 4 hours, then add 200μm sodium carbonate (5.4g, 50.55mmol), and add compound C (8.1g, 40.4mol) at the same time, react at 55-60°C for 6h, add 60ml of pure water after the reaction, precipitate solid, filter , the wet filter cake was dissolved in 80ml of dichloromethane, washed with water, dried with anhydrous sodium sulfate, filtered, concentrated to 20ml under reduced pressure, added with 80ml of n-hexane, precipitated solid, filtered, and dried to obtain Linagliptin intermediate E17.5g , the yield was 90.8%, and the purity was 99.5%.
[0057] Dissolve intermediate E (15g, 26.2mmol) in 150ml of dichloromethane, cool to below 10°C, add 37.5ml of trifluoroacetic acid dropwise, after the dropwise completion, react at room temperature for 5 hours, add dropwise an aqueous potassium carbonate solution with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com