Preparation method for crystal form of linagliptin
A technology of crystal form and seed crystal, which is applied in the field of preparation of linagliptin crystal form, can solve the problems of difficult removal of impurities, increase of economic cost, pressure of waste liquid treatment, unreasonable use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
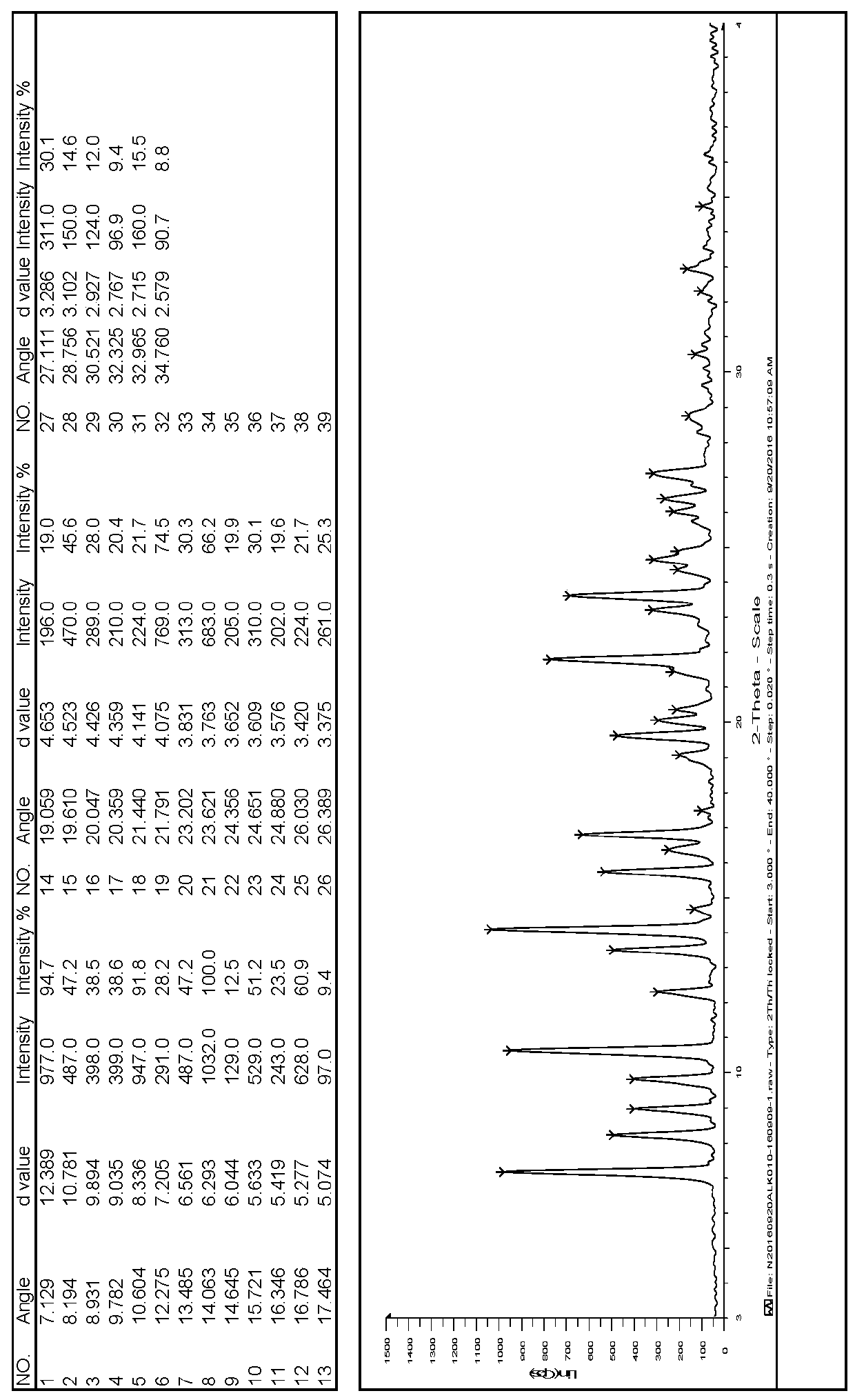
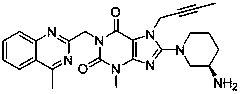
[0021] The 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4- Methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione crude product (purity of 98.66% by HPLC normalization) 10.00g, dissolved in 100ml ethanol, heated to 60~65℃ , Stir until completely dissolved. After stirring for 10 minutes, the temperature was lowered to an internal temperature of 40°C, and seed crystals were added to the clear liquid, and the system quickly became turbid. Continue to cool down to room temperature, stir at room temperature for 2 hours, then stir at 0~5°C for 1 hour, filter, and vacuum dry the filter cake at 50°C for 6 hours to obtain 8.69g of solid powder of the target crystal form (purity by HPLC normalization method: 99.53%) ).
Embodiment 2
[0023] The 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4- Methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione crude product (purity 98.66% by HPLC normalization) 5.00g, dissolved in 20ml methanol, heated to 45~50℃ , Stir until completely dissolved. After stirring for 10 minutes, the temperature was lowered to an internal temperature of 35°C, and seed crystals were added to the clear liquid, and the system became turbid quickly. Continue to cool down to room temperature, stir at room temperature for 2 hours, then stir at 0~5°C for 2 hours, filter, and dry the filter cake under vacuum at 50°C for 6 hours to obtain 3.08g of solid powder of the target crystal form (purity 99.62% by HPLC normalization method) ).
Embodiment 3
[0025] The 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4- Methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione crude product (purity 98.66% by HPLC normalization) 5.00g, dissolved in 75ml isopropanol, heated to 60~ 65°C, stir until completely dissolved. After stirring for 10 minutes, the temperature was lowered to an internal temperature of 45°C, seed crystals were added to the clear liquid, and the system became turbid quickly. Continue to cool down to room temperature, stir at room temperature for 2 hours, then stir at 0~5°C for 1 hour, filter, and vacuum dry the filter cake at 50°C for 6 hours to obtain 3.80g of solid powder of the target crystal form (purity 99.47% by HPLC normalization method) ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com