Linagliptin new crystal form and preparation method thereof
A technology of crystal form and crystal water, which is applied in the field of new crystal form of linagliptin and its preparation, can solve problems such as inability to adapt to large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of amorphous crude product:
[0040] 20g 8-(3R)-amino-piperidin-1-yl-7-but-2-yne-3-methyl-1-((4-methylquinazolin-2-yl)-methyl) - Dissolve the solid xanthine in 100 mL of dichloromethane, fully stir to dissolve, and then concentrate to dryness to obtain 20 g of the amorphous form of Liagliptin, namely the crude amorphous product.
Embodiment 1
[0041] Embodiment 1: Preparation of linagliptin crystal form H
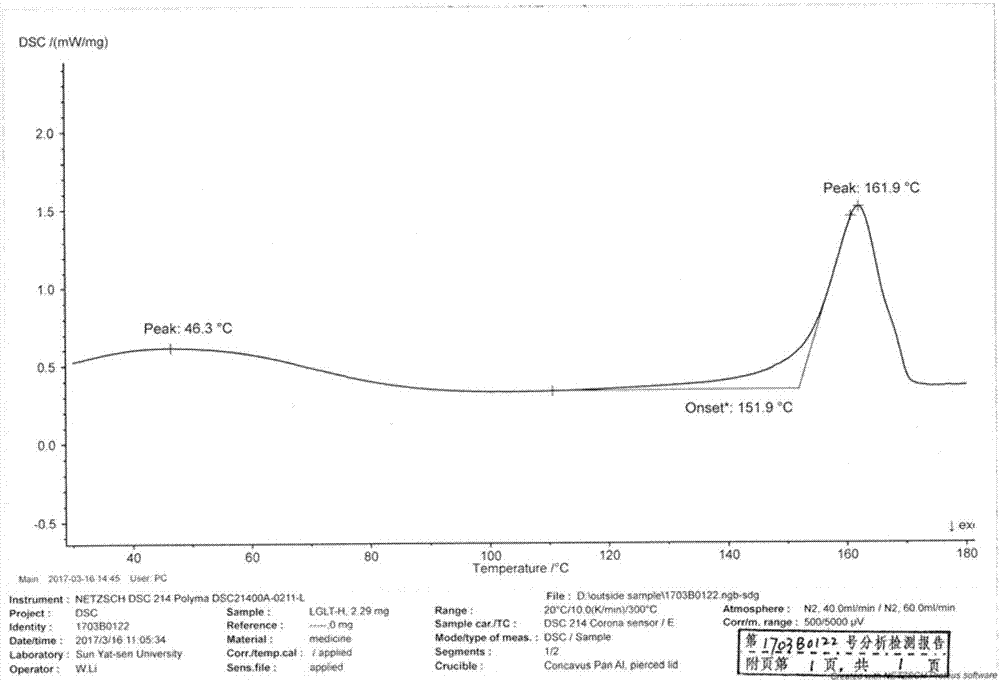
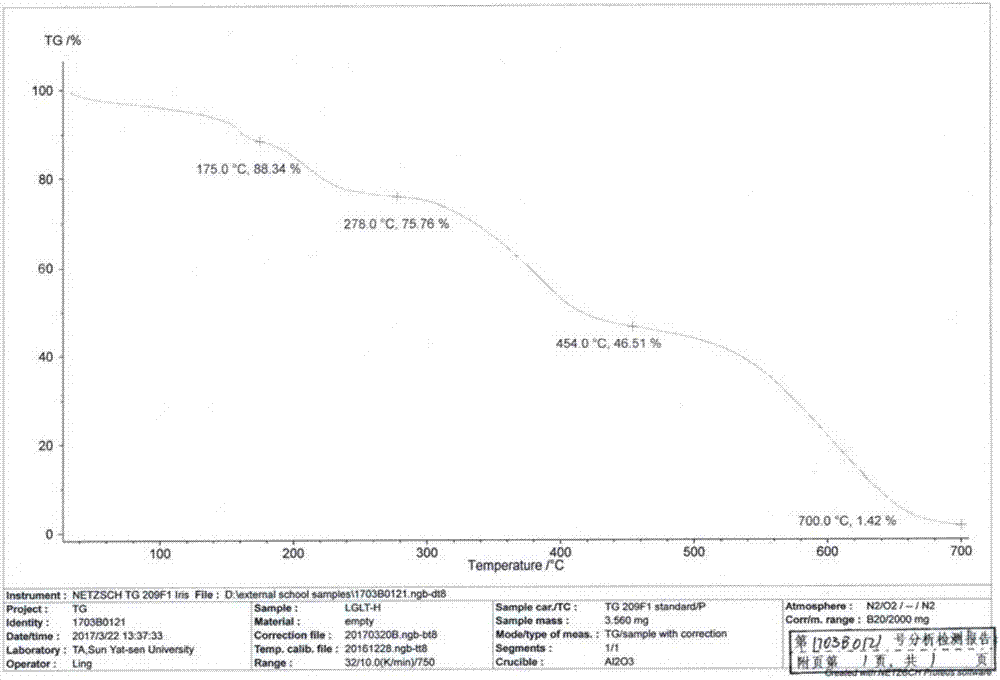
[0042] Take 5 g of the prepared amorphous crude product, add 10 mL of dimethyl sulfoxide, heat to 50 ° C to dissolve, take it out and cool to room temperature, add 10 mL of dioxane under slow stirring, and after solid is precipitated, lower the temperature by 0 to 5 ° C, Stirring was continued for 1 h. Filter and dry under vacuum at 45°C to obtain 3.2 g of off-white crystals. The yield is 64%, the HPLC purity is 98.78%, and the moisture is 0.3%. After determination, its XRD pattern and figure 1 Basically the same; its infrared spectrum and figure 2 Basically the same; the melting range measured by the melting point apparatus is: 150 ° C ~ 153 ° C; its DSC spectrum and image 3 Basically the same; its TGA spectrum is the same as Figure 4 Basically the same; observed under a microscope, the crystal form H is as Figure 5 shown.
Embodiment 2
[0043] Embodiment 2: Preparation of linagliptin crystal form H
[0044] Take 2 g of the prepared amorphous crude product, add 10 mL of dimethyl sulfoxide, heat to 40 ° C to dissolve, take it out and cool to room temperature, add 5 mL of dioxane under slow stirring, and cool down to 0 to 5 ° C after solids are precipitated. Stirring was continued for 1 h. Filter and dry under vacuum at 45°C to obtain 1 g of off-white crystals. The yield is 50%, the HPLC purity is 98.99%, and the moisture is 0.6%. After determination, its XRD pattern and figure 1 Basically the same; its infrared spectrum and figure 2 Basically the same; the melting range measured by the melting point apparatus is: 150 ° C ~ 153 ° C; its DSC spectrum and image 3Basically the same; its TGA spectrum is the same as Figure 4 Basically the same; observed under a microscope, the crystal form H is as Figure 5 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com