Simple preparation method of II-type antidiabetic drug linagliptin
A simple and simple technology for diabetes, which is applied in the field of diabetes, can solve the problems of complicated operation, too many raw materials, and long time consumption, and achieve the effect of cheap and easy-to-obtain raw materials, simple processing and operation, simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 3-Methyl-7-(2-butyn-1-yl)-8-bromo-xanthine (2.7 g, 9 mmol) was added to 2-chloromethyl-4-methylquinazoline (2.1 g, 10.8mmol) and potassium carbonate (1.9g, 13.5mmol) in DMSO (30ml) solution, under the catalysis of potassium iodide (0.015g, 0.09mmol), react at 70 ℃ for 7 hours, then add potassium carbonate (1.9g , 13.5mmol), while adding (R)-3-aminopiperidine (1.1g, 10.8mmol), reacted at 70°C for 8 hours with a one-pot method, and added twice the amount of saturated saline ( 60ml), the solid was precipitated, and the crude product (4.1g) was obtained by suction filtration. The crude product was recrystallized with methanol (4.5ml), filtered by suction, and dried to obtain the pure product 8-[(3R)-3-aminopiperidine-1 - Base] -7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-1H-purine- 2,6-Diketone (3.8 g).
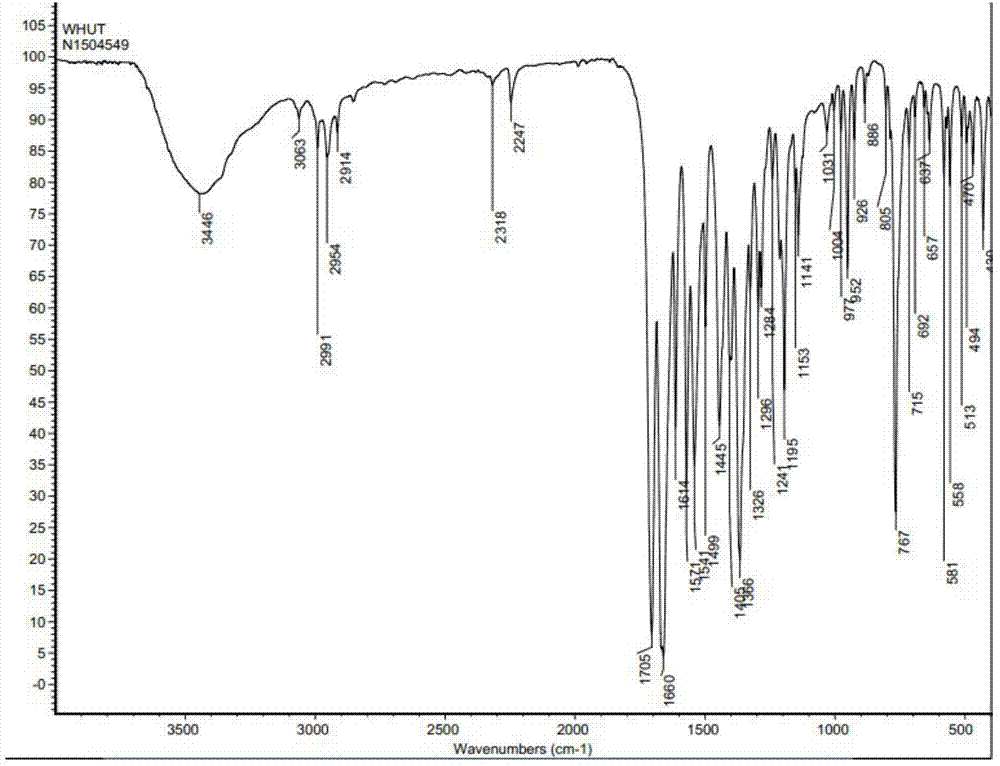
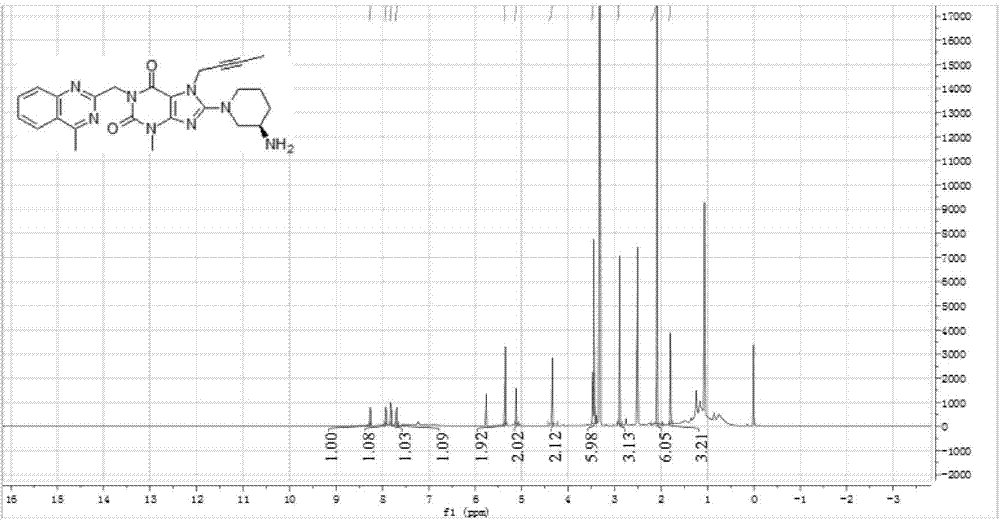
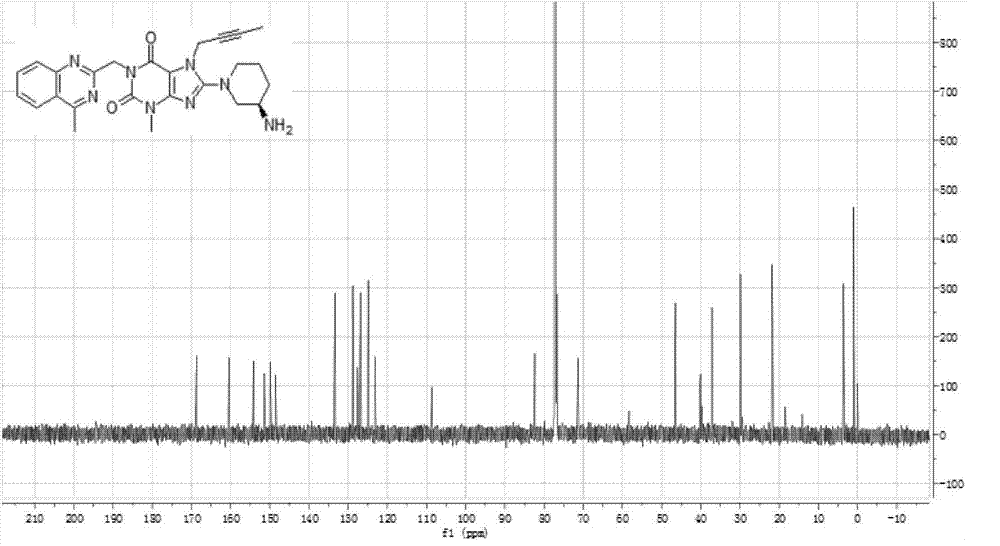
[0038] The infrared absorption spectrogram (IR spectrum testing instrument: BrukerVECTOR-22, test condition: KBr tablet) of the product obtain...
Embodiment 2
[0043] 3-Methyl-7-(2-butyn-1-yl)-8-bromo-xanthine (8.1 g, 27 mmol) was added to 2-chloromethyl-4-methylquinazoline (6.3 g, 32.4mmol) and potassium carbonate (5.7g, 40.5mmol) in DMSO (90ml) solution, under the catalysis of potassium iodide (0.045g, 0.27mmol), react at 80 ℃ for 7 hours, then add potassium carbonate (5.7g , 40.5mmol), while adding (R)-3-aminopiperidine (3.3g, 32.4mmol), reacted at 80°C for 8 hours with a one-pot method, and added twice the amount of saturated saline ( 180ml), precipitated a solid, and filtered it with suction to obtain the crude product (12.4g). The crude product was recrystallized with methanol (13ml), filtered with suction, and dried to obtain the pure product 8-[(3R)-3-aminopiperidine-1- Base] -7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-1H-purine-2 , 6-diketone (11.9 g).
Embodiment 3
[0045] 3-Methyl-7-(2-butyn-1-yl)-8-bromo-xanthine (81 g, 270 mmol) was added to 2-chloromethyl-4-methylquinazoline (63 g, 324 mmol) and potassium carbonate (57g, 405mmol) in DMSO (900ml) solution, under the catalysis of potassium iodide (0.45g, 2.7mmol), react at 70°C for 8 hours, then add potassium carbonate (57g, 405mmol), and add (R)-3-aminopiperidine (33g, 324mmol) was reacted at 70°C for 7 hours in a one-pot method, and two times the amount of saturated saline (1800ml) was added after the reaction was completed, and a solid was precipitated, filtered by suction, The crude product (121g) was obtained, and the crude product was recrystallized with methanol (125ml), filtered and dried to obtain the pure product 8-[(3R)-3-aminopiperidin-1-yl]-7-(2-butyne yl)-3,7-dihydro-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-1H-purine-2,6-dione (115 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com