Olanzapine-containing biodegradable microsphere preparation and preparation method thereof
A technology of biodegradation and olanzapine, which is applied in the direction of drug combination, neurological diseases, bulk delivery, etc., can solve the problems that cannot meet the clinical application, and achieve the improvement of drug release in vitro, particle size distribution, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
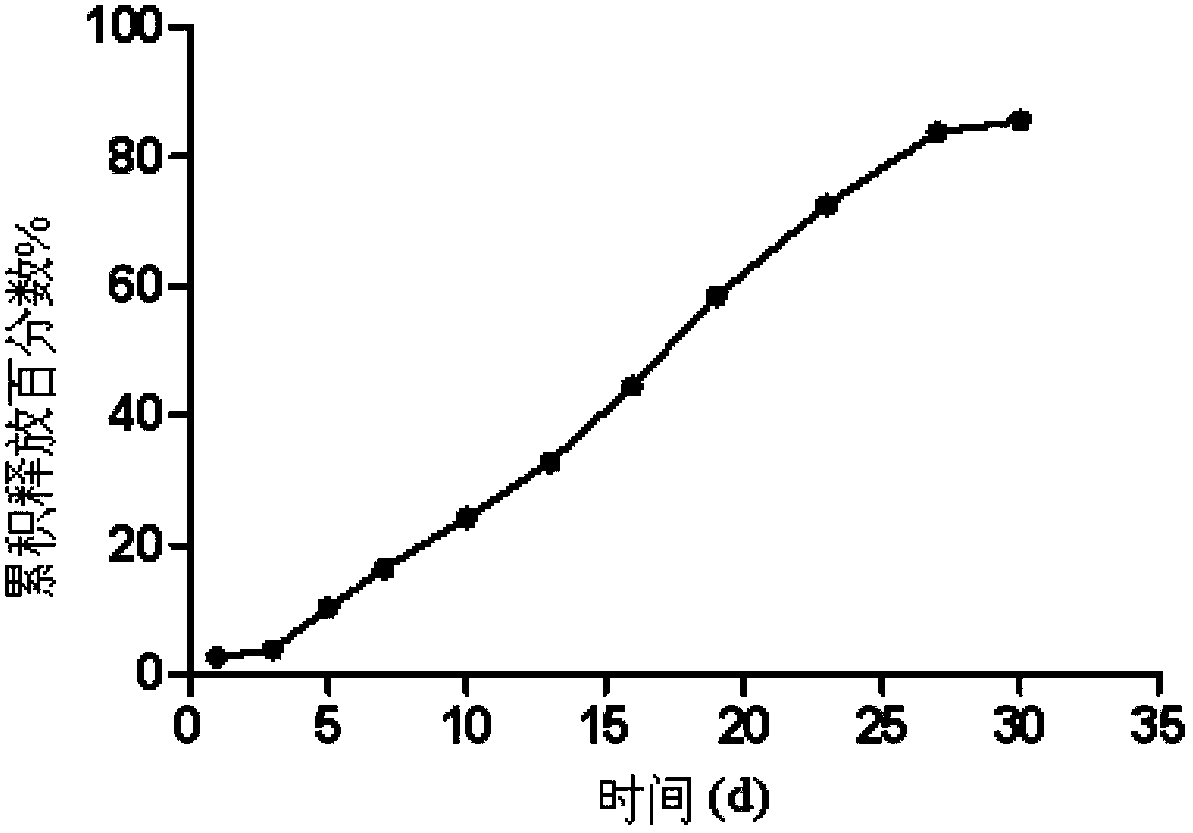
Embodiment 1
[0055] Preparation of olanzapine microspheres:
[0056] (1) Weigh 0.3g of PLGA (weight average molecular weight 6,5124, LA:GA is 75:25, molar ratio) and 0.2g of olanzapine dissolved in 6.5g of dichloromethane and N~methyl~pyrrolidone mixed solvent ( Volume ratio 1:9), add 0.05g macrogol glyceride oleate, mix to obtain oil phase;
[0057] (2) Weigh 5g of PVA in 500g of distilled water, heat to dissolve, cool to room temperature, add 1g of NaCl and stir to dissolve, adjust the pH of the water phase to 7.5 with NaOH, add a stirrer, and keep it at 15°C under water bath conditions;
[0058] Among them, the degree of alcoholysis of PVA is 90%, the average number of polymer chains is 1700, and the weight average molecular weight is 20,000;
[0059] Slowly inject the oil phase into the water phase under the liquid surface, stir and emulsify for 1 hour, replace the supernatant, add ethanol, the weight content of ethanol in the water phase is 20wt%, and after magnetic stirring at room ...
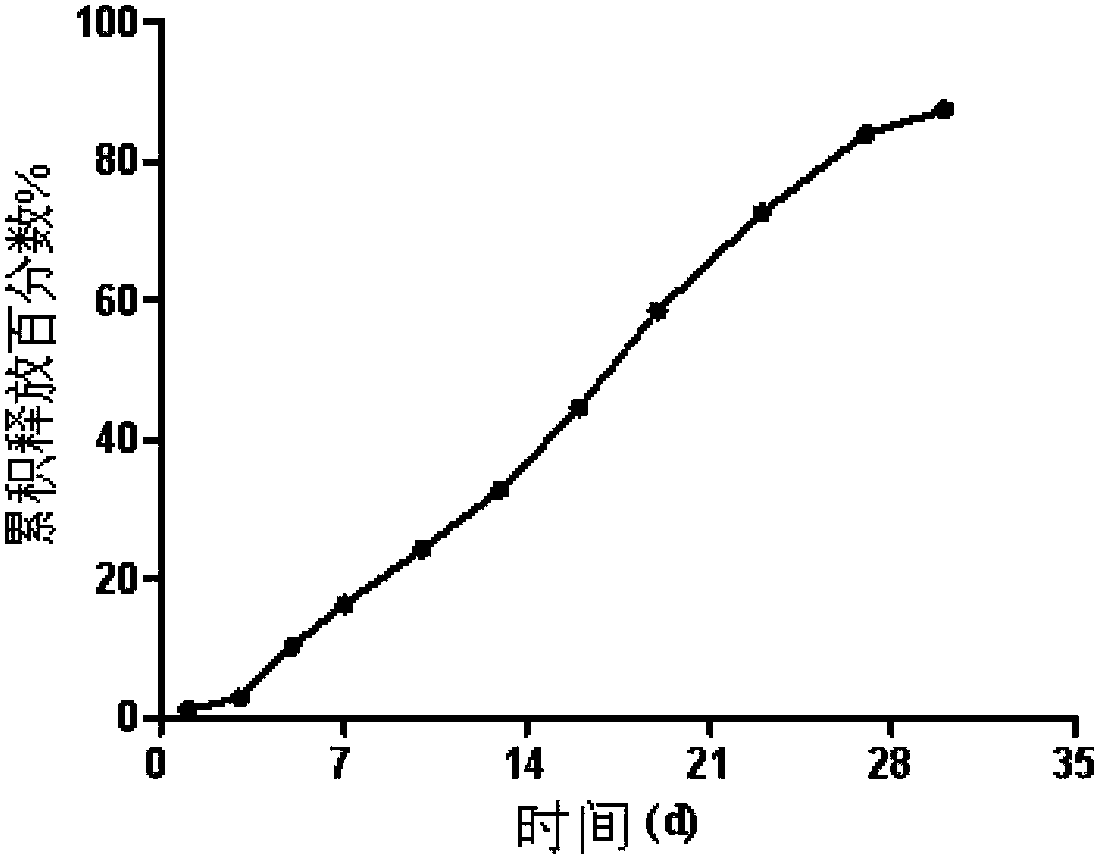
Embodiment 2
[0075] Preparation of olanzapine microspheres
[0076] Weigh 0.3 g of PLGA (molecular weight 9,5726, LA:GA 75:25) and 0.2 g of olanzapine dissolved in 5 g of dichloromethane and N-methyl-pyrrolidone mixed solvent (volume ratio 1:2), add 0.1 g glyceryl tricaprylate, mixed to obtain an oily phase;
[0077] Weigh 4g of PVA in 400g of distilled water, dissolve, cool to room temperature, add 1.5g of NaCl and stir to dissolve, adjust the pH of the water phase to 7.5 with NaOH, add a stirrer, and keep it at 20°C under water bath conditions;
[0078] The average alcoholysis degree of PVA is 87%, the average polymer chain number is 1750, and the weight average molecular weight is 10,000;
[0079] Inject the oil phase into the water phase under the liquid surface, stir and emulsify for 1 hour, replace the supernatant, add ethanol, the content of ethanol in the water phase is 20wt%, magnetically stir at room temperature for 1 hour, replace the supernatant, and continue stirring for 24 h...
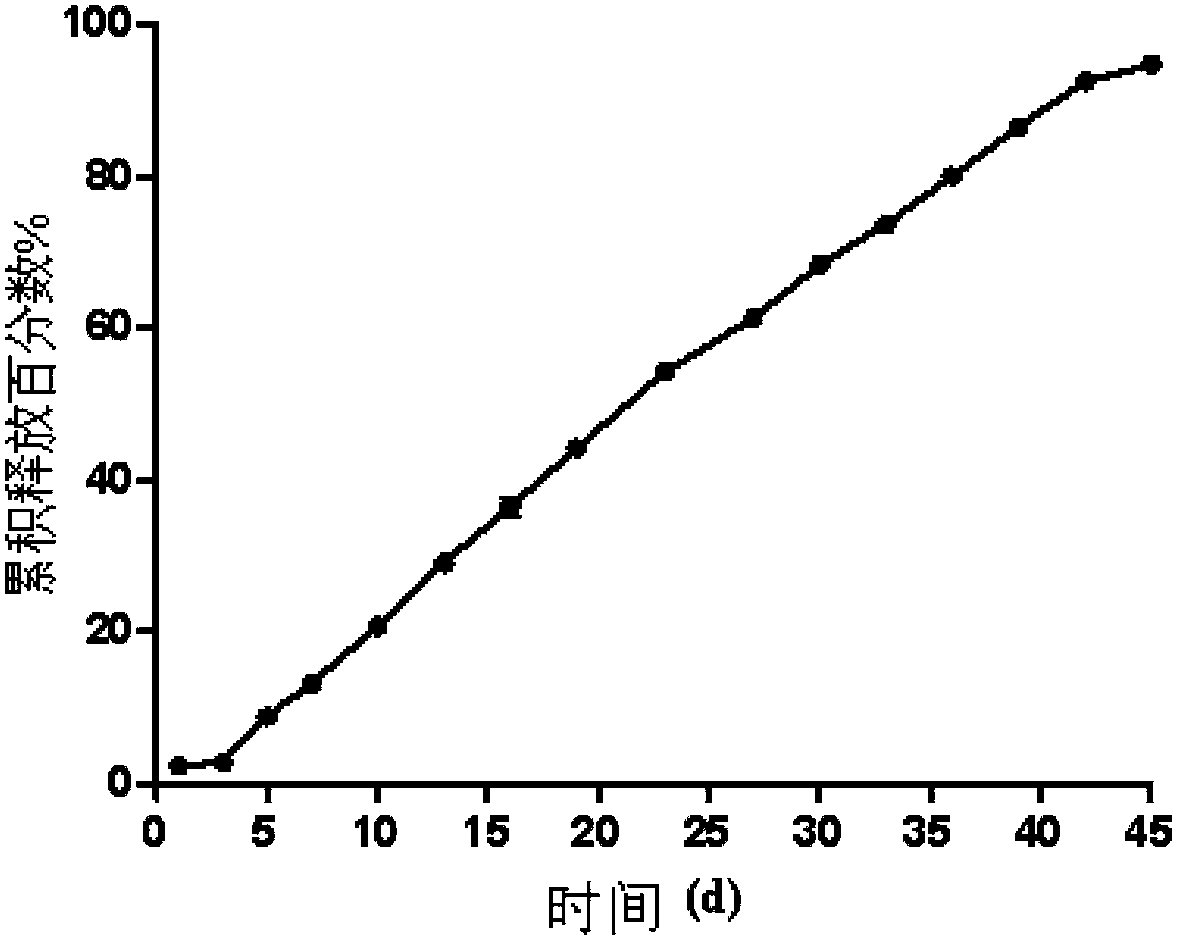
Embodiment 3
[0088] Preparation of olanzapine microspheres
[0089] Weigh 0.275g of PLGA (molecular weight 10,5328, LA:GA 75:25) and 0.225g of olanzapine dissolved in 5g of dichloromethane and N~methyl~pyrrolidone mixed solvent (volume ratio 1:9), add 0.1 g dimyristoylphosphatidylcholine (DMPC) to obtain the oil phase;
[0090]Weigh 6g of PVA in 600g of distilled water, dissolve, cool to room temperature, add 1.5g of NaCl, stir to dissolve, adjust the pH of the water phase to 7.5 with NaOH, add a stirrer, and keep it at 15°C under water bath conditions;
[0091] The average alcoholysis degree of PVA is 88%, the average polymer chain number is 1720, and the weight average molecular weight is 10,500;
[0092] Inject the oil phase into the water phase under the liquid surface, stir and emulsify for 1 hour, replace the supernatant, add ethanol, the content of ethanol in the water phase is 30wt% ethanol, magnetically stir at room temperature for 1 hour, replace the supernatant, and continue st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com