Olanzapine composition and preparation method thereof
A technology for olanzapine and its composition, which is applied in the field of olanzapine composition and its preparation, can solve the problems of reduced water solubility and stability, and achieve the effects of fewer auxiliary materials, easy scale-up production, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
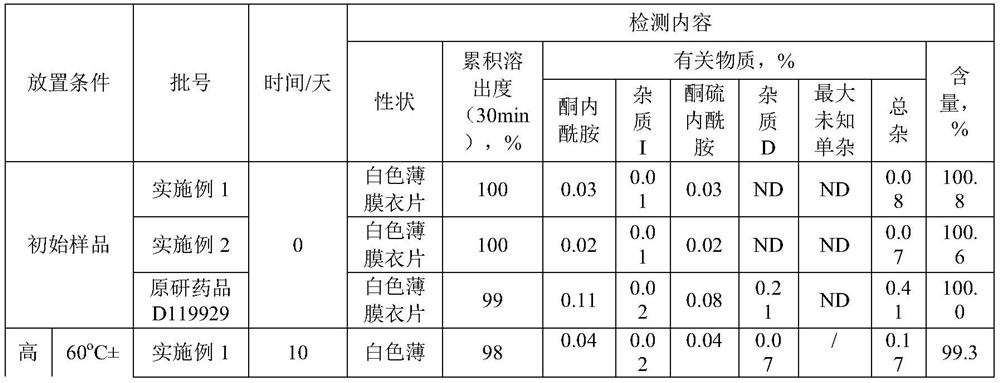
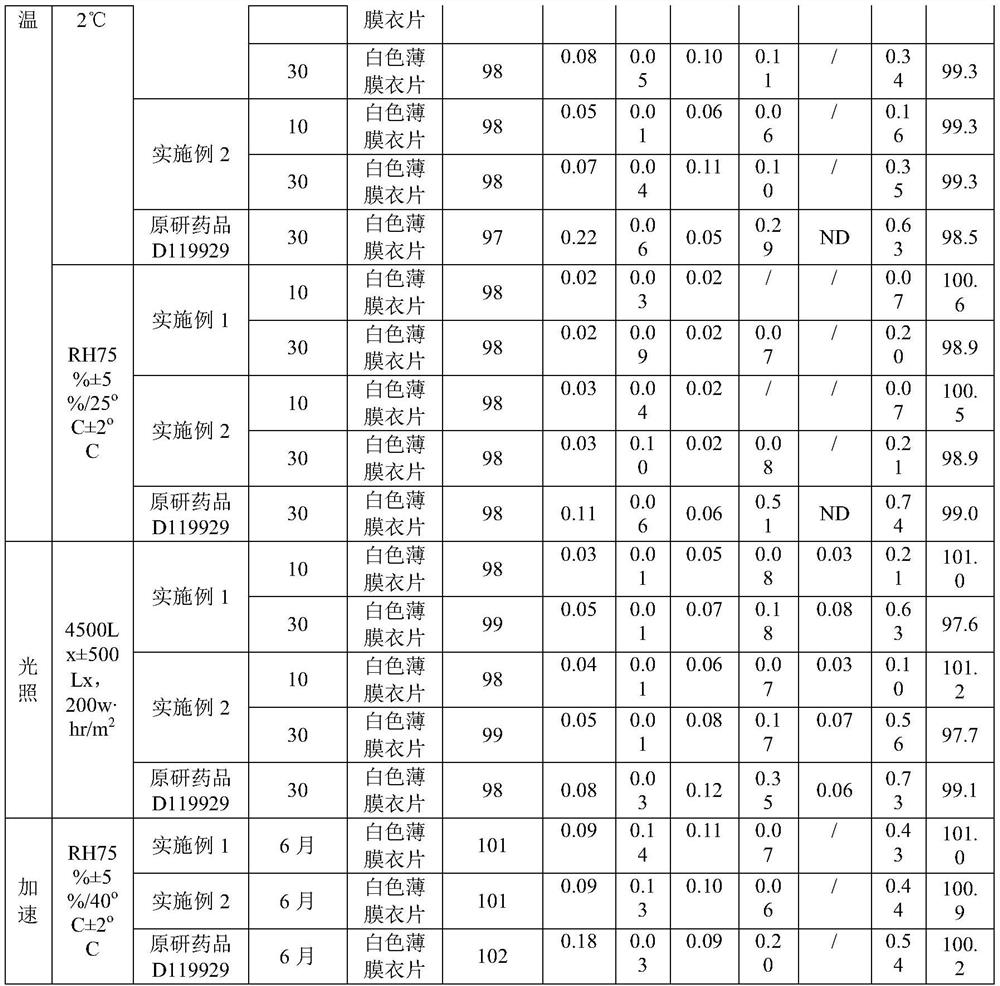
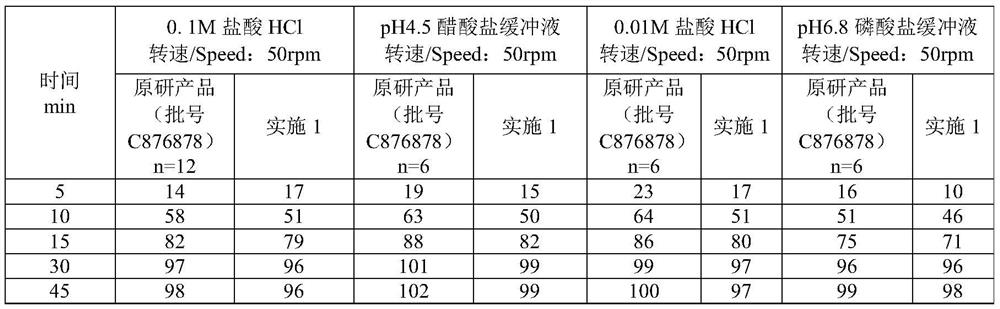
Embodiment 1
[0033] A kind of preparation method method of olanzapine composition, when it produces 10,000, comprise the steps:
[0034] (1) Pretreatment: Take olanzapine, lactose microcrystalline cellulose, and low-substituted hydroxypropyl cellulose to pass through 40 meshes respectively, and set aside; wherein the particle size of olanzapine is 4.58 μm-317 μm, and low-substituted hydroxypropyl cellulose Base cellulose, its content is 4%;
[0035] (2) Weighing: Olanzapine 0.1kg, MICROCELAC R 100 is 3.834kg, low-substituted hydroxypropyl cellulose is 0.125kg, magnesium stearate is 0.041kg;
[0036] (3) Mixing: put lactose microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and olanzapine in a mixer in sequence, and mix for 40 minutes at a speed of 15 r / min;
[0037] (4) Dry granulation: dry granulation parameters are: working pressure: 25bar; granulation screen: 0.8mm; make the granule Carr index less than 20%, and the angle of repose less than 30°;
[0038] (5) Lubric...
Embodiment 2
[0045] A kind of preparation method of described olanzapine composition, when it produces 10,000, comprise the steps:
[0046] (1) Pretreatment: Weigh olanzapine, lactose microcrystalline cellulose, and low-substituted hydroxypropyl cellulose to pass through 40 meshes respectively, and set aside; wherein the particle size of olanzapine is 4.58 μm-317 μm, and the low-substituted hydroxypropyl cellulose Propyl cellulose, its content is 4%;
[0047] (2) Weighing: olanzapine 0.05kg, lactose microcrystalline cellulose MICROCELAC R 100 is 1.917kg, low-substituted hydroxypropyl cellulose is 0.063kg, and magnesium stearate is 0.021kg. Weigh each raw material and auxiliary material according to the weight ratio;
[0048] (3) Mixing: put lactose microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and olanzapine in a mixer in sequence, and mix for 20 minutes at a speed of 15 r / min;
[0049] (4) Dry granulation: dry granulation parameters are: working pressure: 25bar; gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com