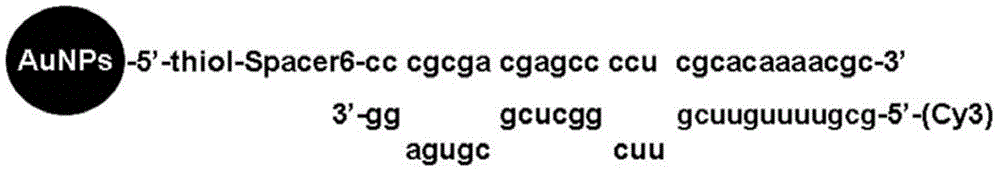Patents
Literature
30results about How to "Good pharmacological properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
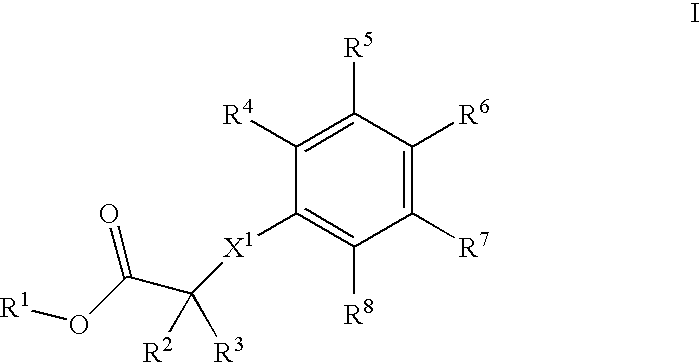
Phenyl derivatives, their manufacture and use as pharmaceutical agents
InactiveUS20050096337A1Improve securityEliminate side effectsBiocideOrganic chemistryEnantiomerMedicinal chemistry
Owner:F HOFFMANN LA ROCHE & CO AG
Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors
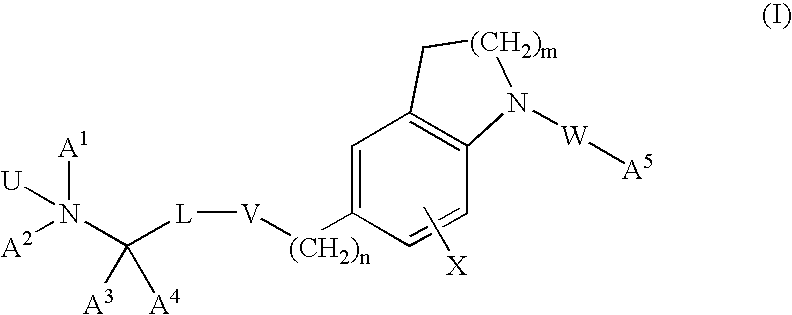
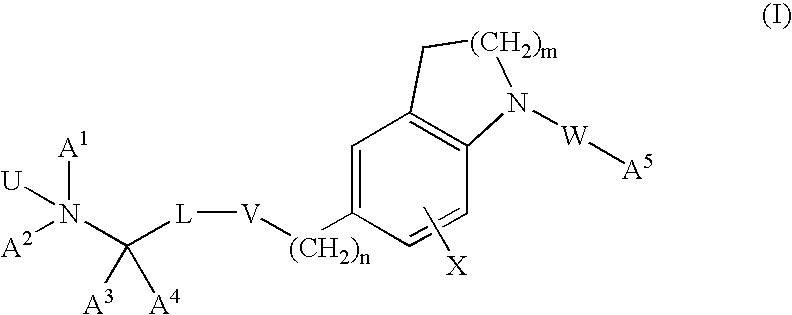
InactiveUS20120302549A1Good pharmacological propertiesImprove propertiesOrganic active ingredientsBiocideProphylactic treatmentPharmacology
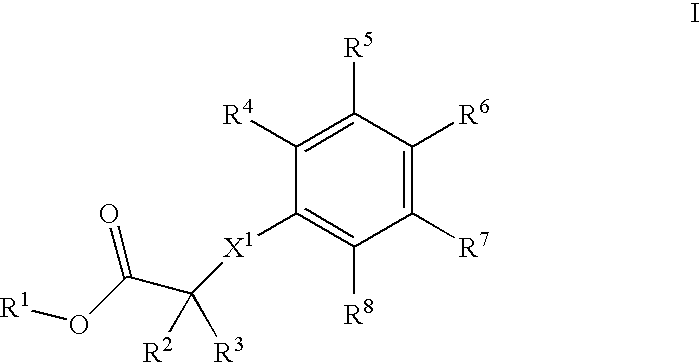
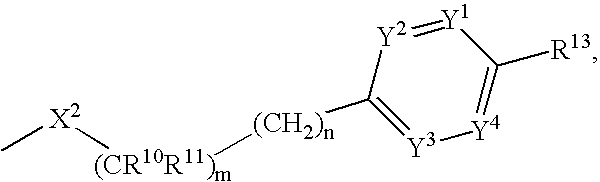
The present invention provides spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines of formula Ihaving BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors
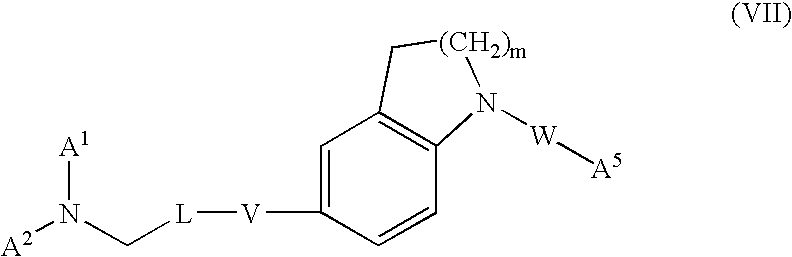
InactiveUS20130072478A1Good pharmacological propertiesSolution value is not highOrganic active ingredientsNervous disorderDiseaseProphylactic treatment
The present invention provides N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl)-amides of formula Ihaving BACE1 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease.
Owner:F HOFFMANN LA ROCHE & CO AG
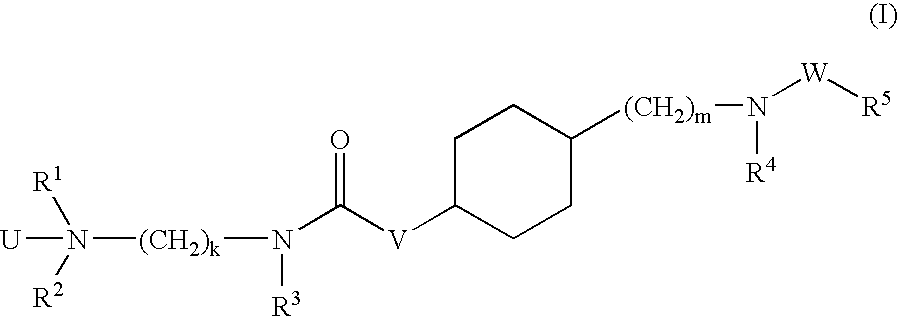
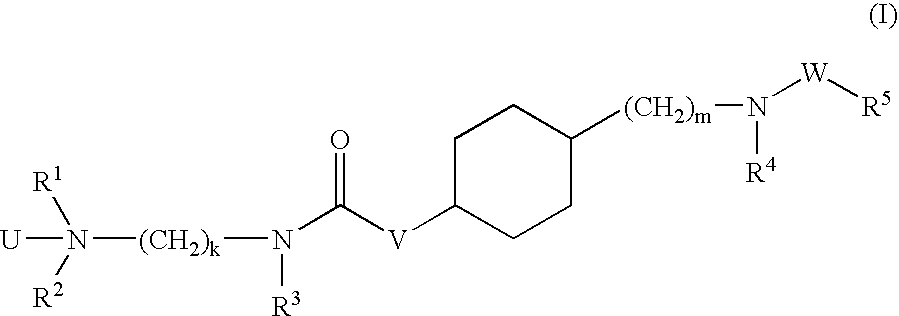
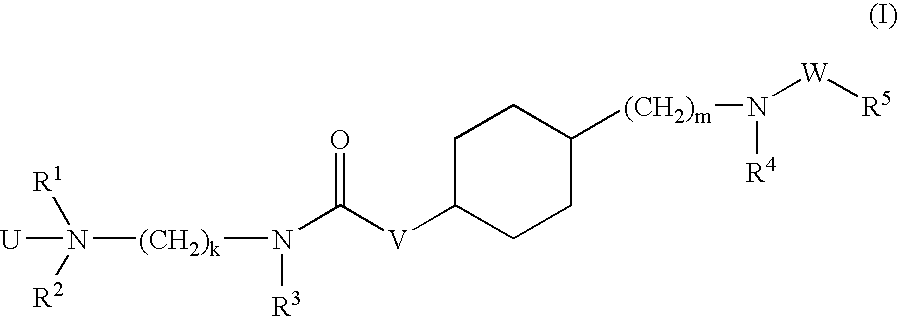
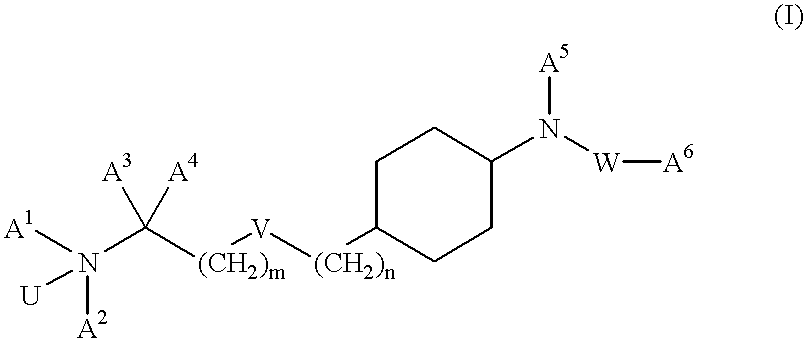
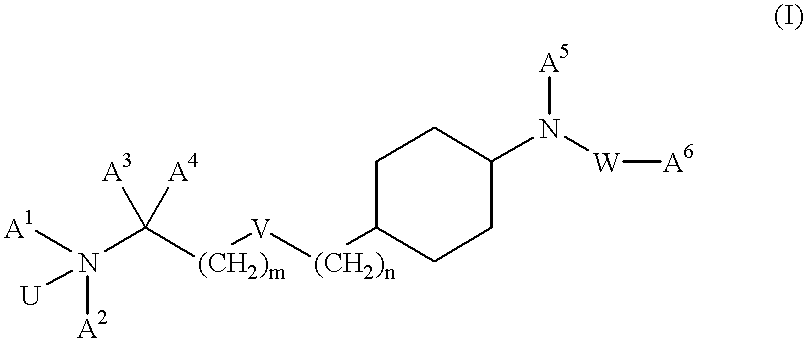
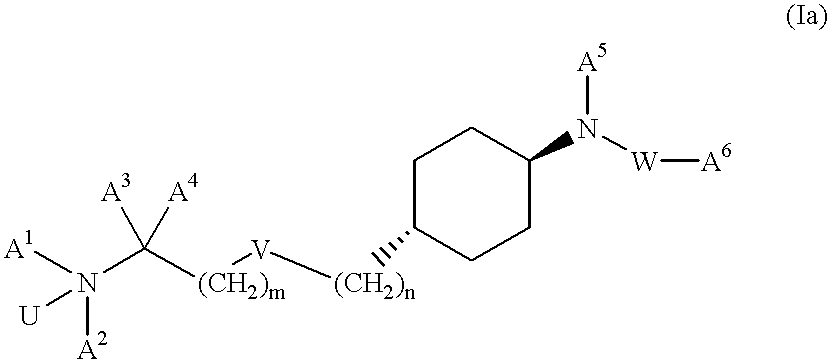
Aminoalkylamide substituted cyclohexyl derivatives
InactiveUS20050065210A1Reduce plasma cholesterol levelImprove glucose toleranceBiocideAntimycoticsAbnormal tissue growthCholesterol cholelithiasis
The present invention relates to compounds of formula I wherein R1, R2, R3, R4, R5, U, V, W, k and m are as defined in the description and claims, and pharmaceutically acceptable salts and / or pharmaceutically acceptable esters thereof. The compounds are useful for the treatment and / or prophylaxis of diseases which are associated with 2,3-oxidosqualene-lanosterol cyclase such as hypercholesterolemia, hyperlipemia, arteriosclerosis, vascular diseases, mycoses, parasit infections, gallstones, tumors and / or hyperproliferative disorders, and treatment and / or prophylaxis of impaired glucose tolerance and diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
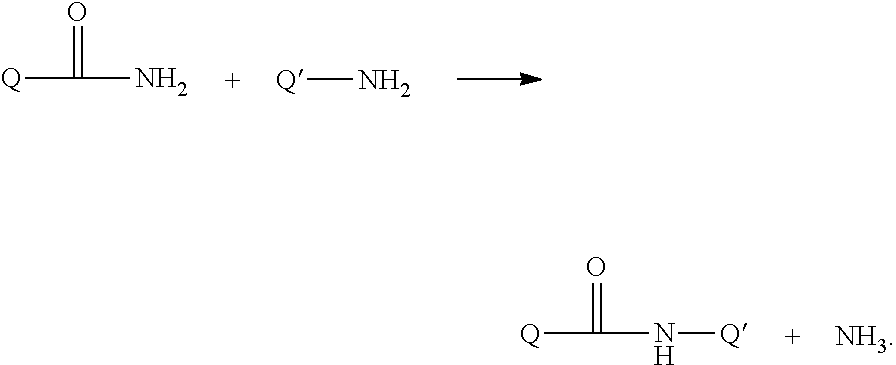
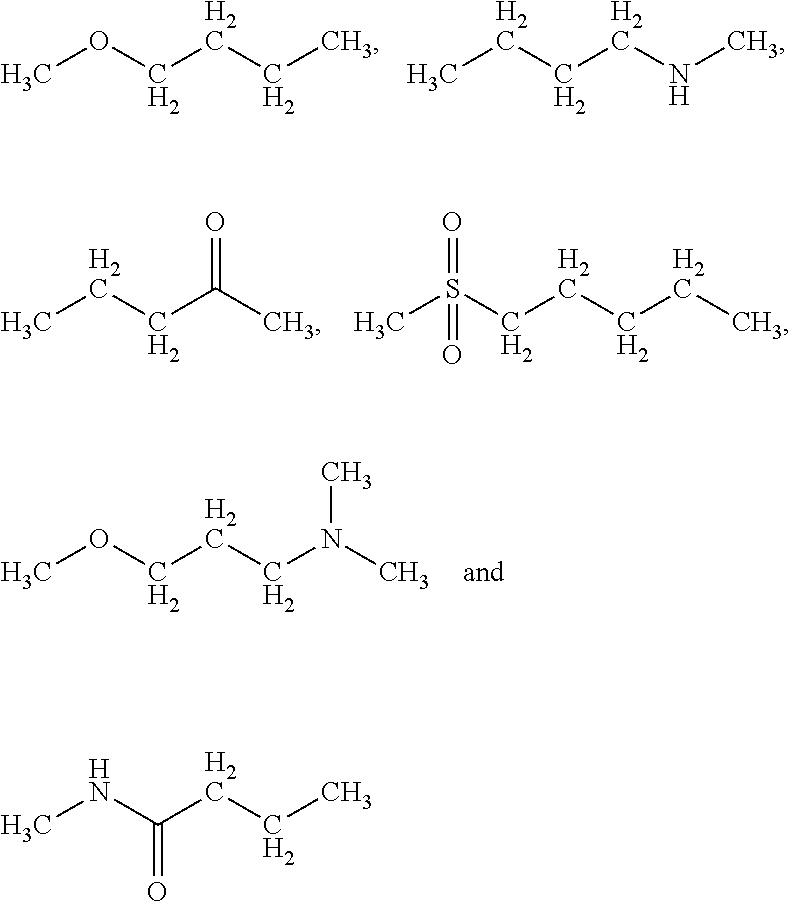
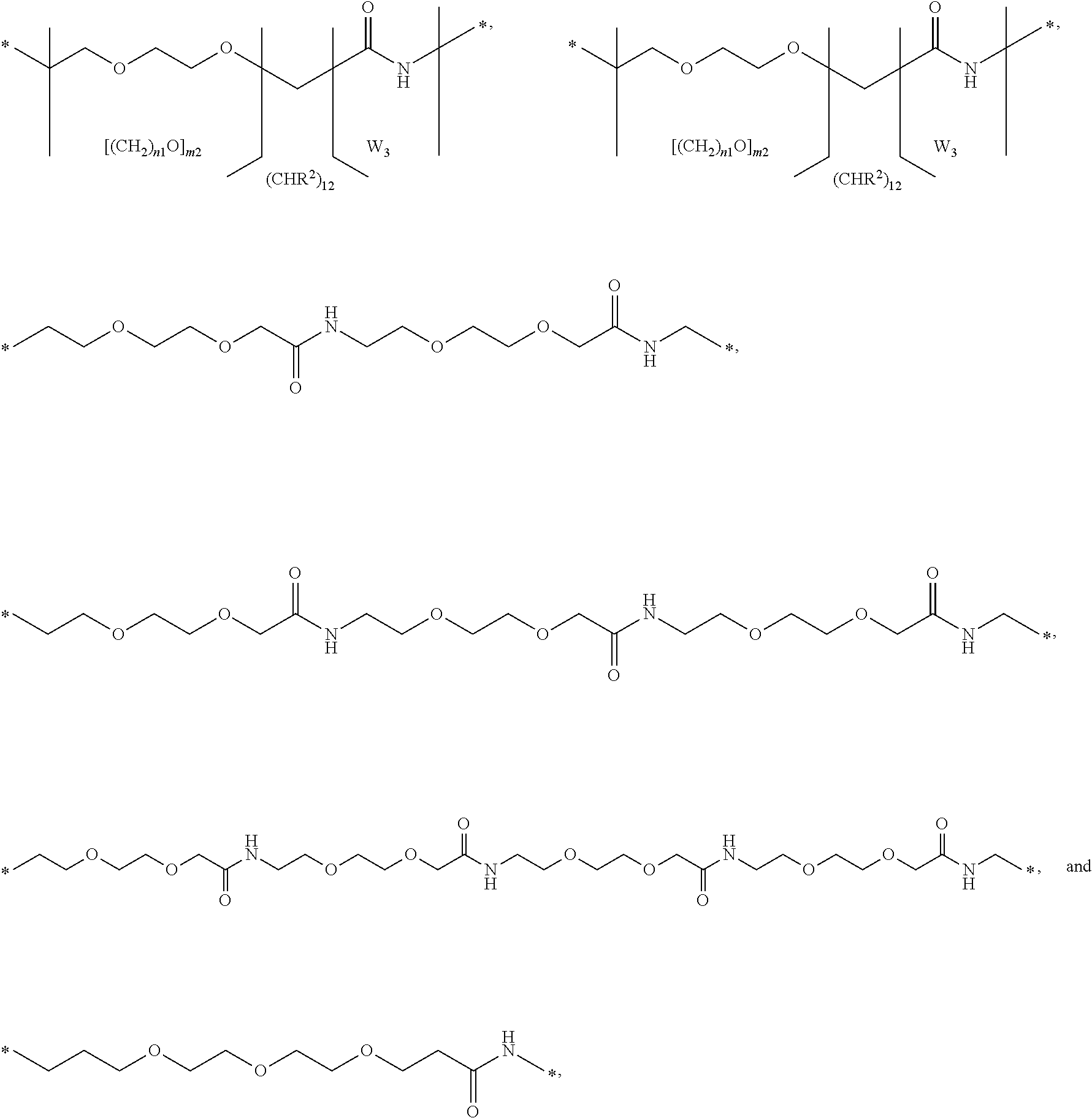
Growth hormones with prolonged in-vivo efficacy
The present invention relates to polypeptide compound optimized for subcutaneous administration, exemplified by growth hormone conjugates having a linker providing non-covalent binding to albumin.
Owner:NOVO NORDISK HEALTH CARE AG
Cationic elaioplast and its adenovirus composition, its preparing method and use
ActiveCN101045037AImprove stabilityUnique formulaGenetic material ingredientsPharmaceutical non-active ingredientsCholesterolElaioplast
A cationic liposome is proportionally prepared from DOPE, DOTMA and cholesterol and can be used to encapsulate the object (adenovirus carrier) to obtain a recombinant human endostatin adenovirus-cationic liposome composition carrying the active antineoplastic genes for suppressing the growth of tumor. Its preparing process is also disclosed.
Owner:SICHUAN UNIV
Dihydroindole and tetrahydroquinoline derivatives
InactiveUS20050020624A1Reduce plasma cholesterol levelImprove glucose toleranceBiocideAntimycoticsCyclaseDisease
The present invention relates to novel dihydroindole and tetrahydroquinoline derivatives and pharmaceutically acceptable salts and / or pharmaceutically acceptable esters thereof. The compounds are useful for the treatment and / or prophylaxis of diseases which are associated with 2,3-oxidosqualene-lanosterol cyclase such as hypercholesterolemia, hyperlipemia, arteriosclerosis, vascular diseases, mycoses, gallstones, tumors and / or hyperproliferative disorders, and treatment and / or prophylaxis of impaired glucose tolerance and diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
2,3-oxidosqualene-lanosterol cyclase inhibitors
InactiveUS6858651B2Reduce plasma cholesterol levelImprove glucose toleranceBiocideUrea derivatives preparationCholesterol cholelithiasisEnzyme inhibitor
The present invention relates to aminocyclohexanol derivatives useful for the treatment and / or prophylaxis of diseases which are associated with 2,3-oxidosqualene-lanosterol cyclase such as hypercholesterolemia, hyperlipemia, arteriosclerosis, vascular diseases, mycoses, gallstones, tumors and / or hyperproliferative disorders, and treatment and / or prophylaxis of impaired glucose tolerance and diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
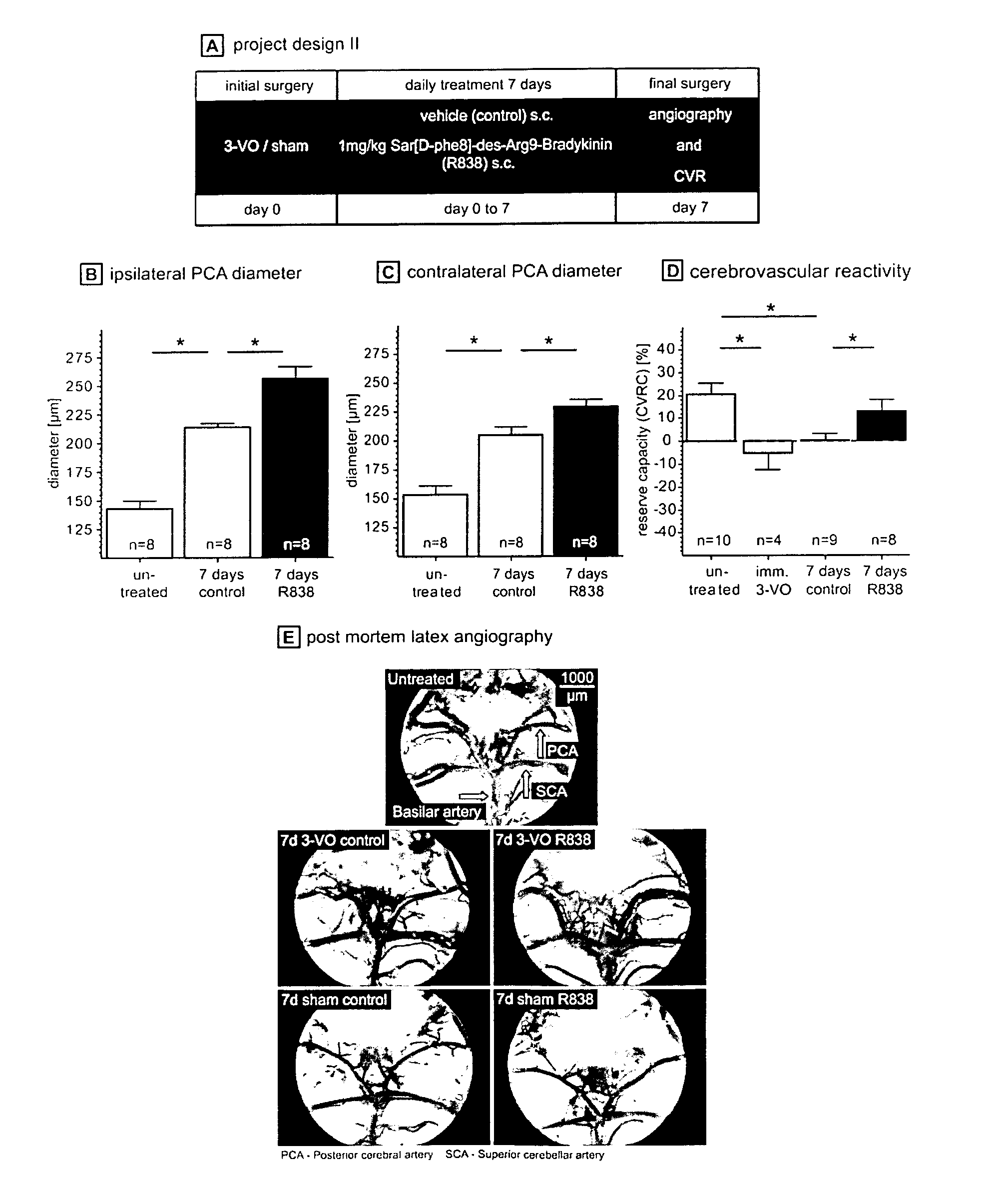
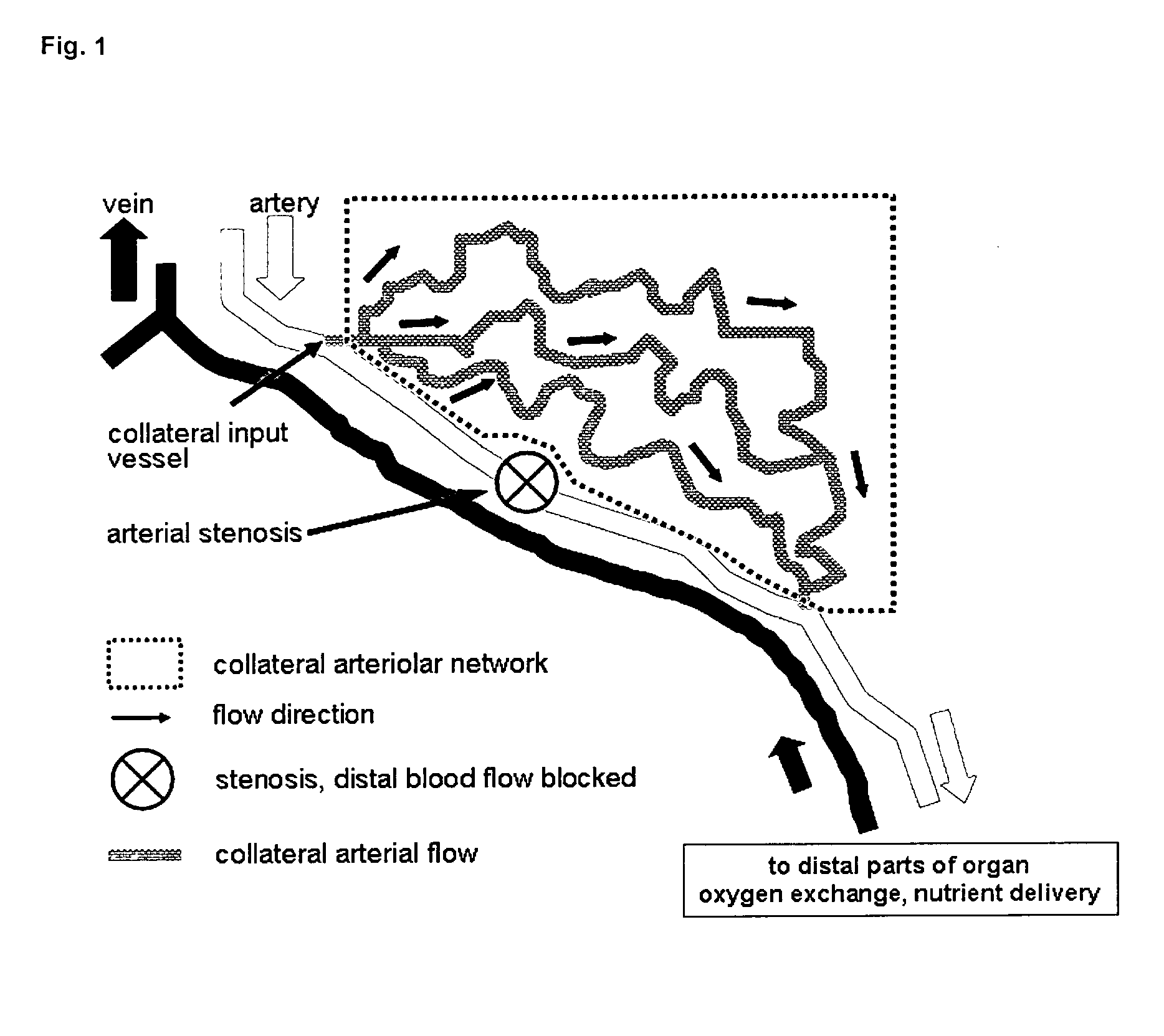
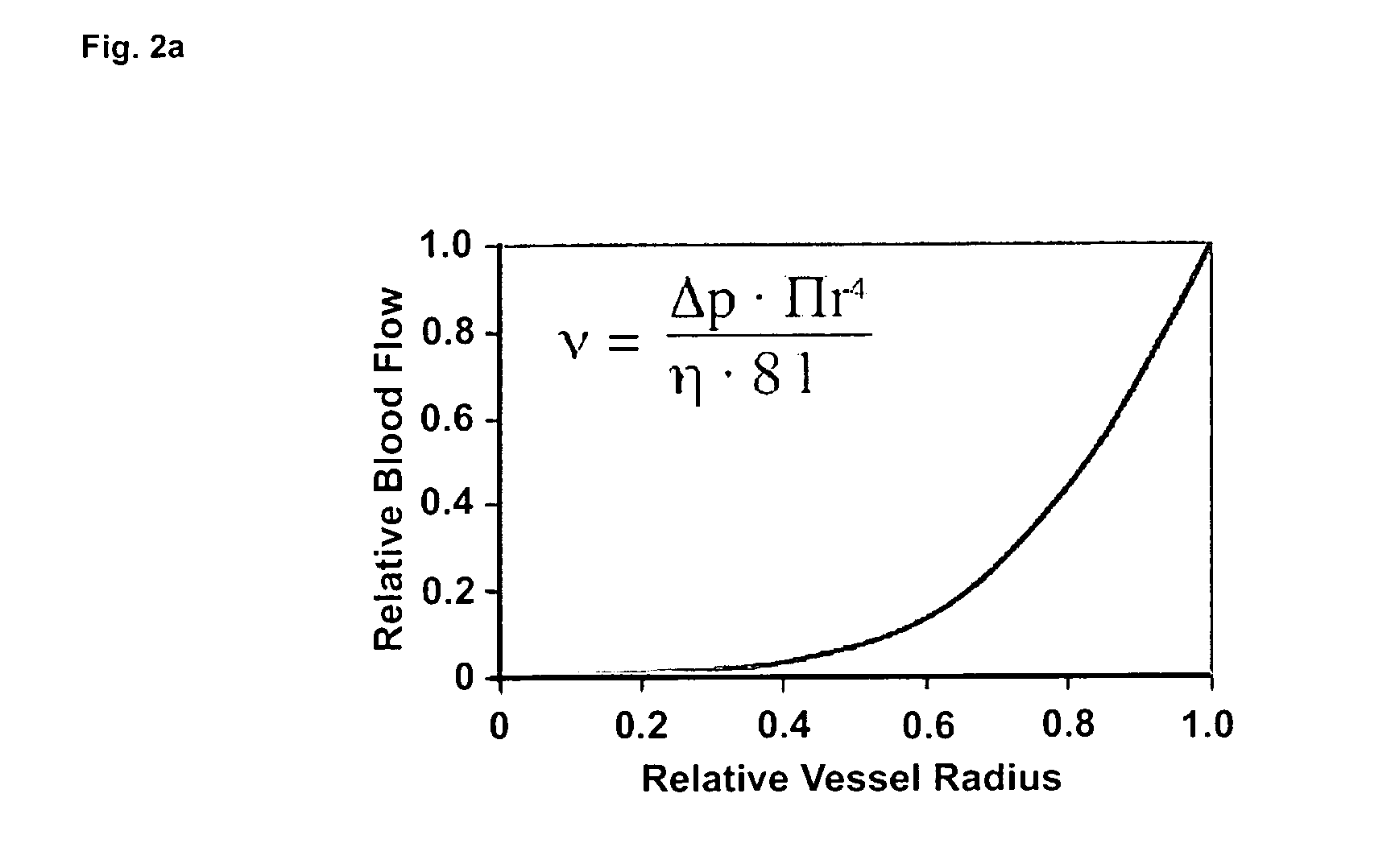
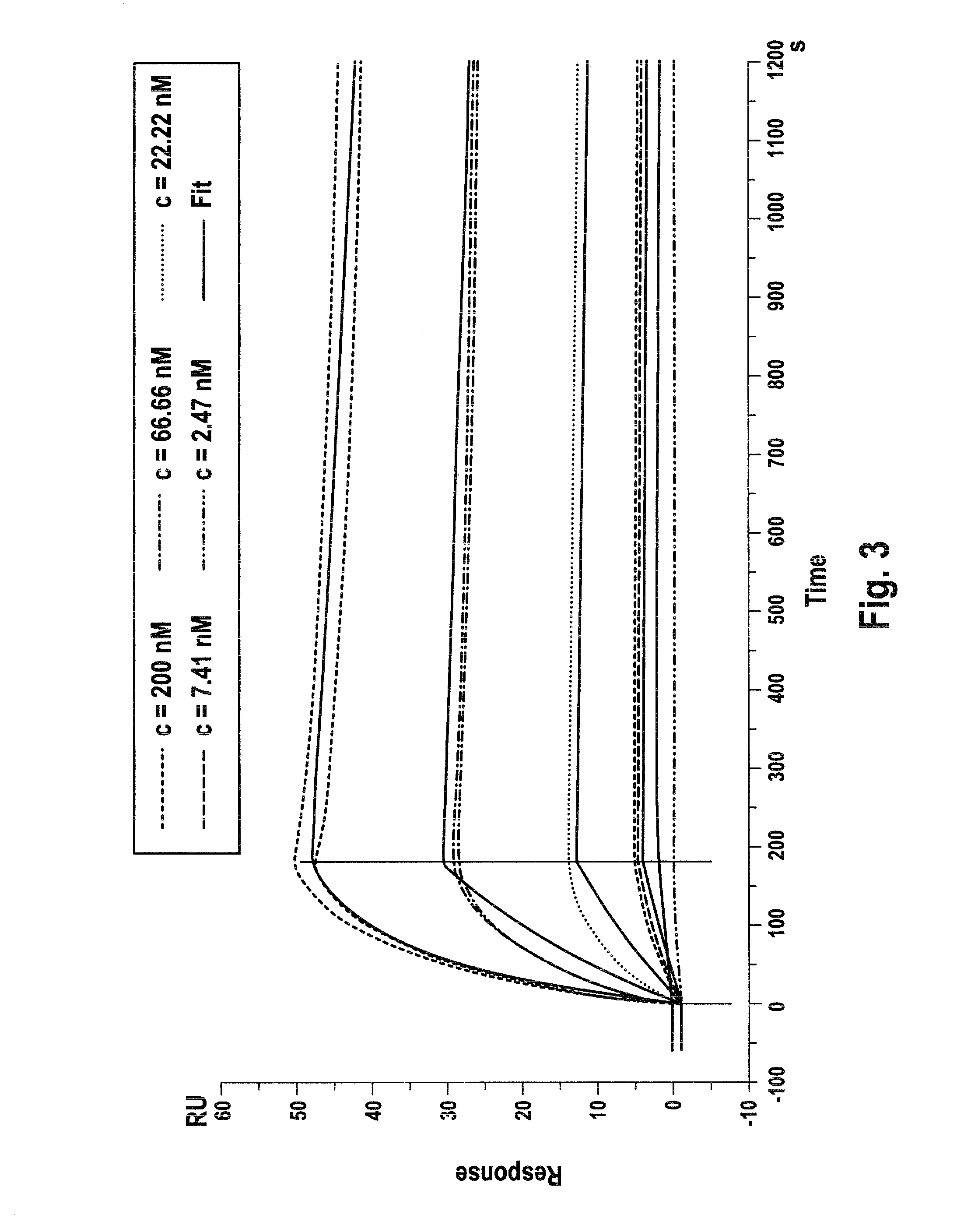
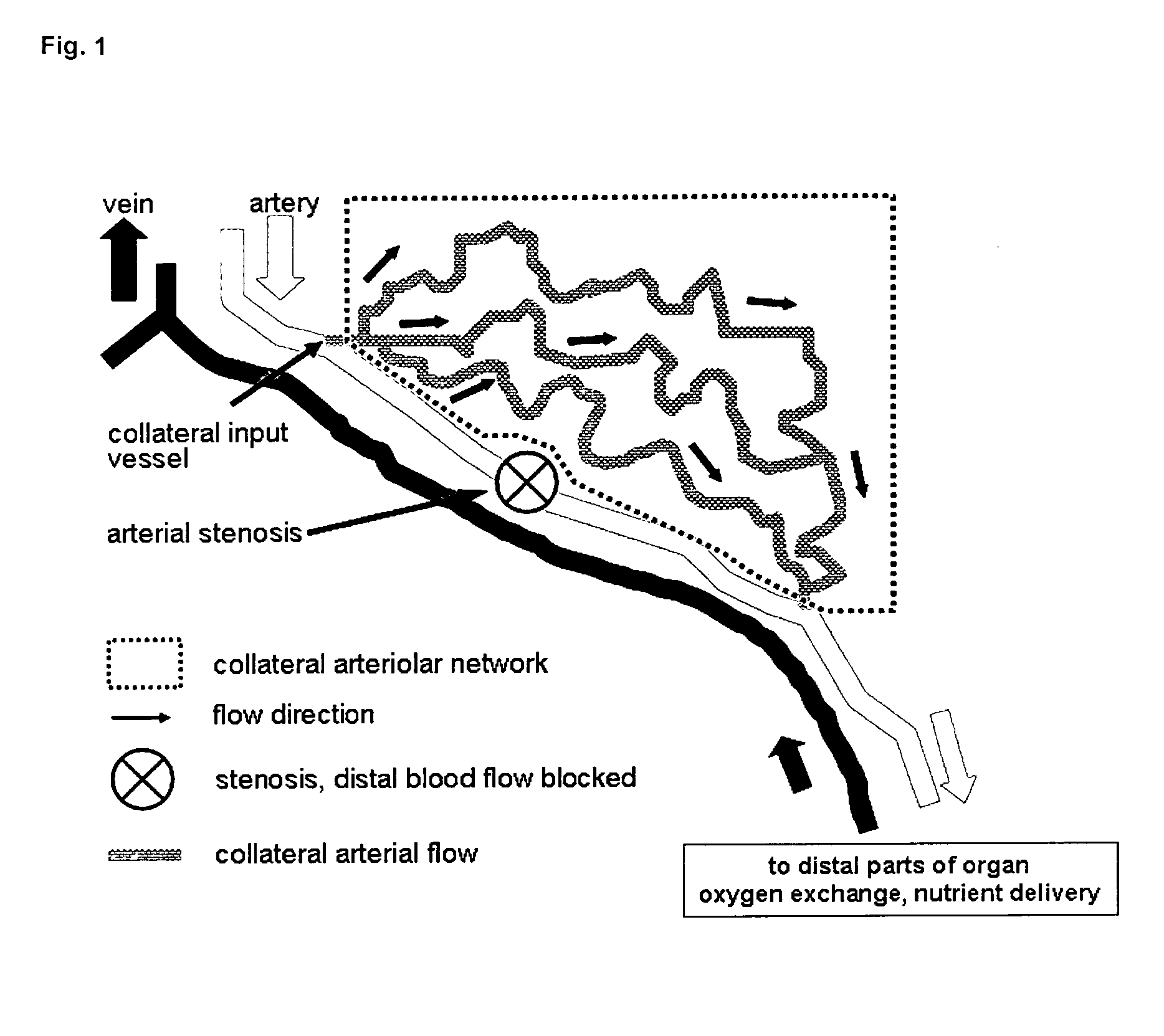
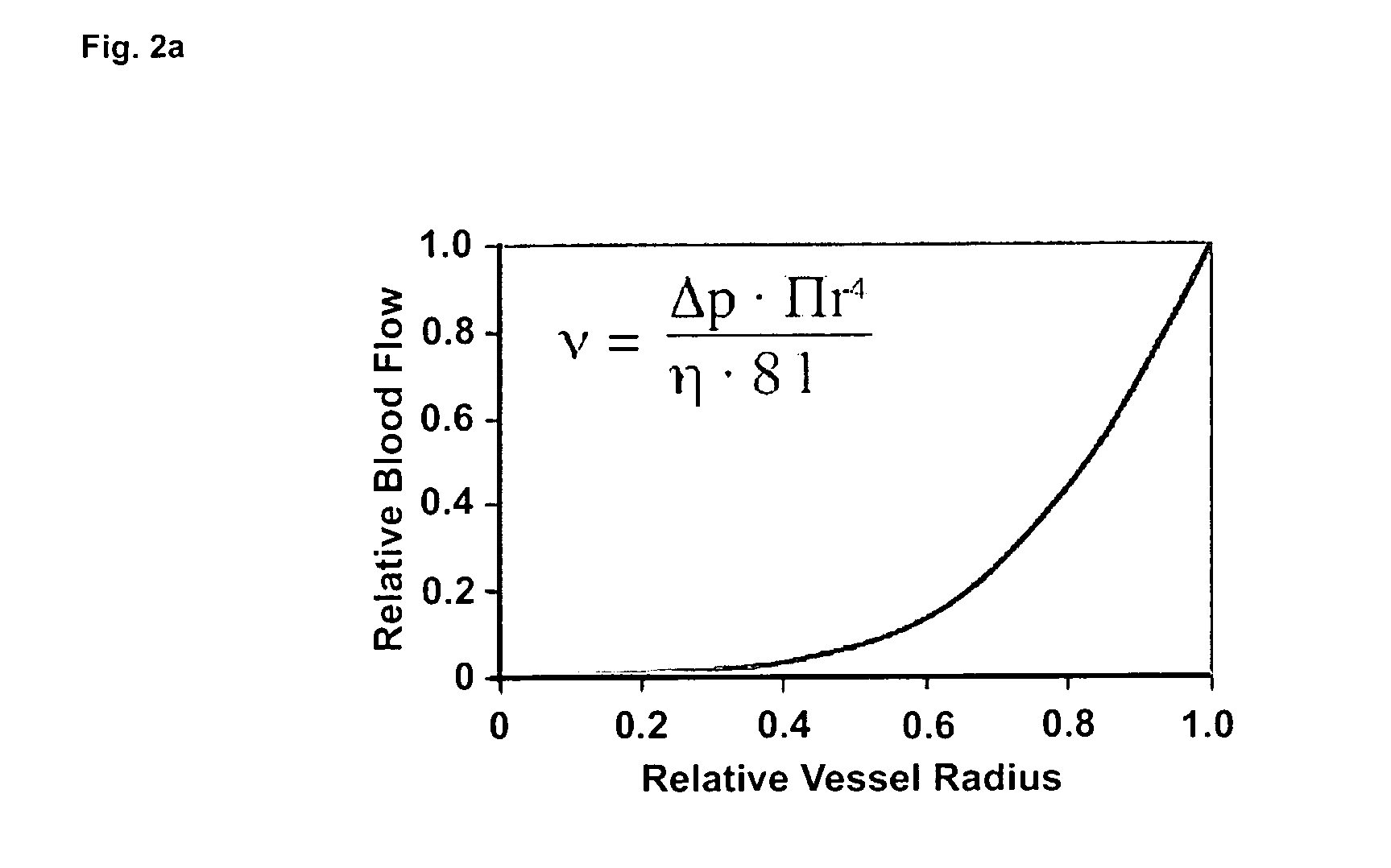
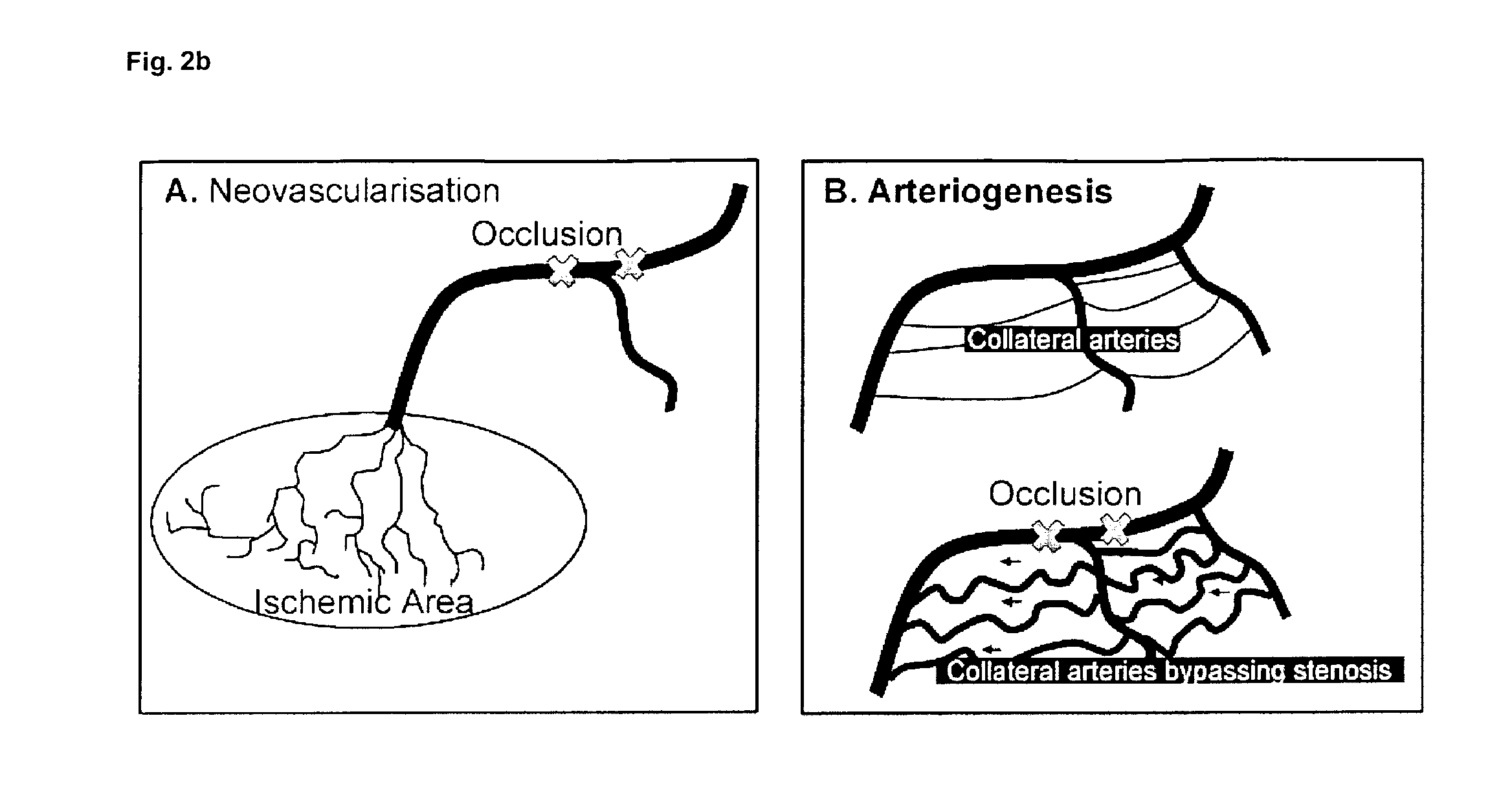
Therapeutic use of agonists or antagonists of bradykinin receptor 1 or 2, for modulation collateral blood vessel growth
ActiveUS20130136717A1Increasing concentration and half-lifeGood pharmacological propertiesOrganic active ingredientsHormone peptidesBradykinin receptorCvd risk
The present invention relates to bradykinin receptor modulators and pharmaceutical compositions thereof for use as a medicament for modulating collateral blood vessel growth of collateral arteries and / or other blood vessels of pre-existing arterial networks. The bradykinin receptor modulators of arteriogenesis are applicable in the treatment and / or prevention of disorders associated with defective blood flow or blood vessel malformation. A preferred aspect of the invention relates to bradykinin receptor agonists for use as a medicament for the prevention of cardiovascular ischemic disease in a patient at risk thereof. Further, the invention relates to a bradykinin receptor agonist for use in a method for treating a cardiovascular ischemic disease in a patient in need thereof, wherein said cardiovascular ischemic disease is a peripheral limb disease.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Growth Hormones with Prolonged In-Vivo Efficacy
The present invention relates to polypeptide compound optimized for subcutaneous administration, exemplified by growth hormone conjugates having a linker providing non-covalent binding to albumin.
Owner:NOVO NORDISK AS
Compositions and associated methods of mesoporous nanoparticles comprising platinum-acridine molecules
InactiveUS20190290685A1Good pharmacological propertiesReduce systemic toxicityOrganic active ingredientsOrganic chemistryLipid formationExocytic vesicle
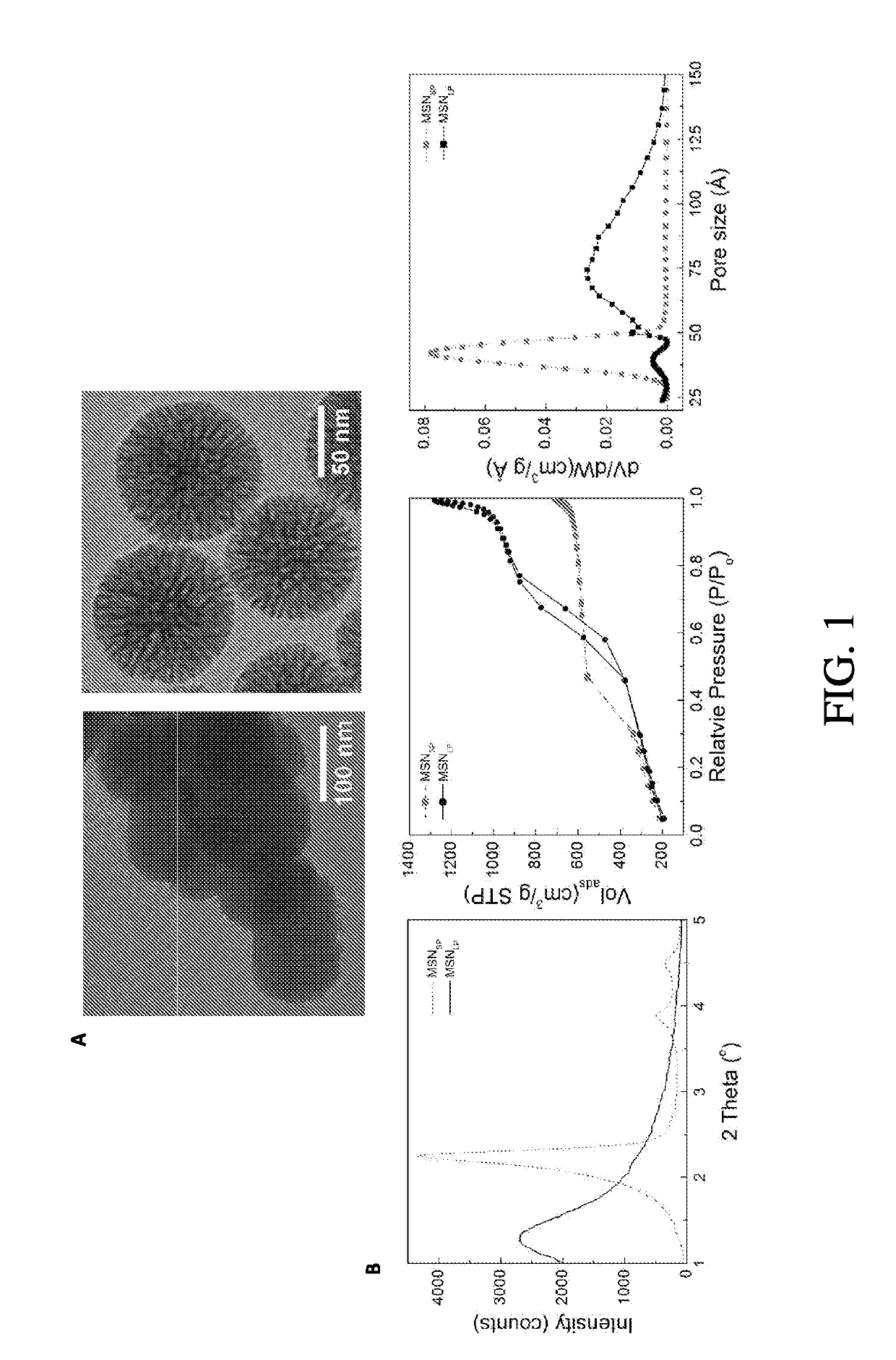
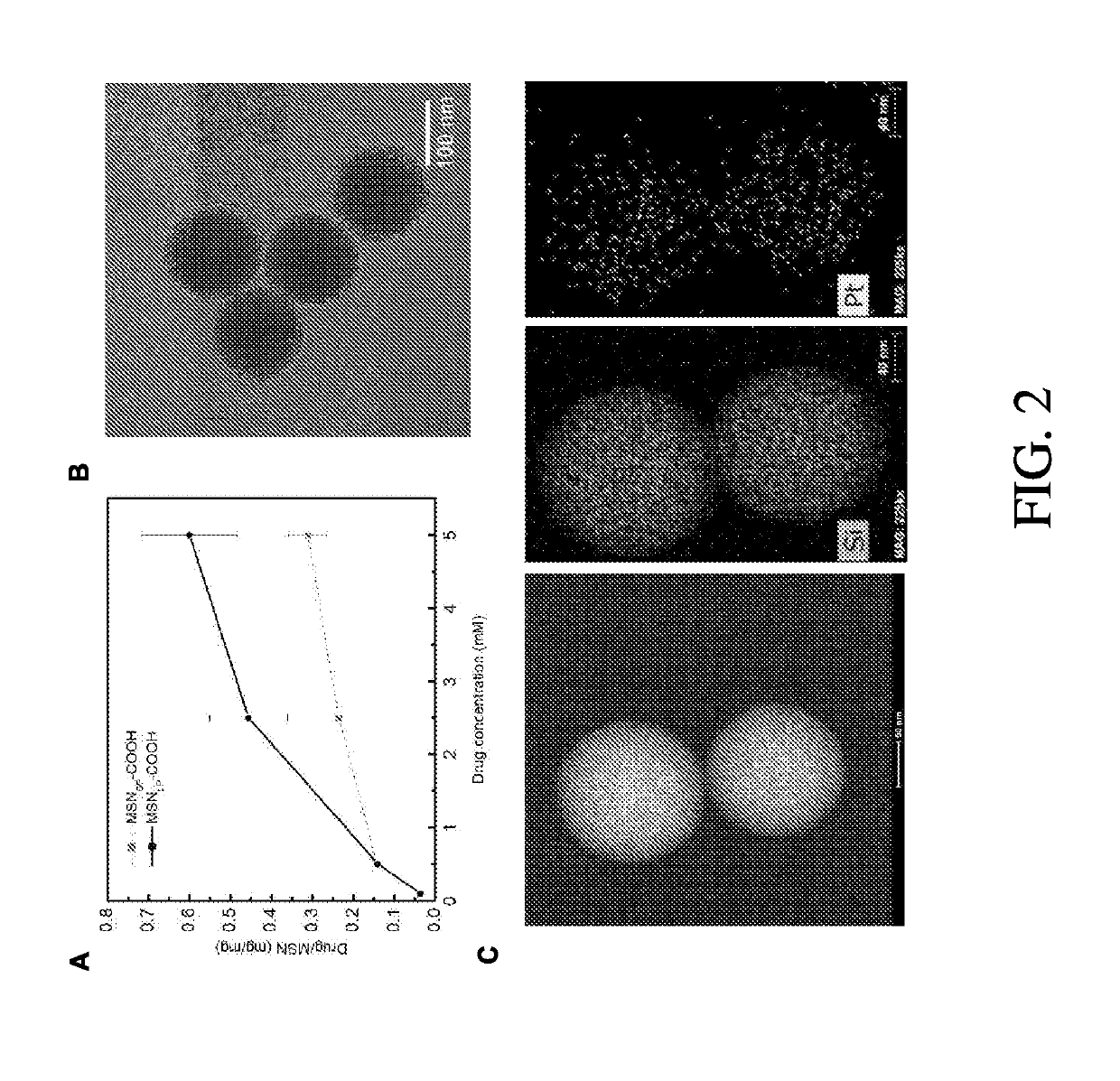
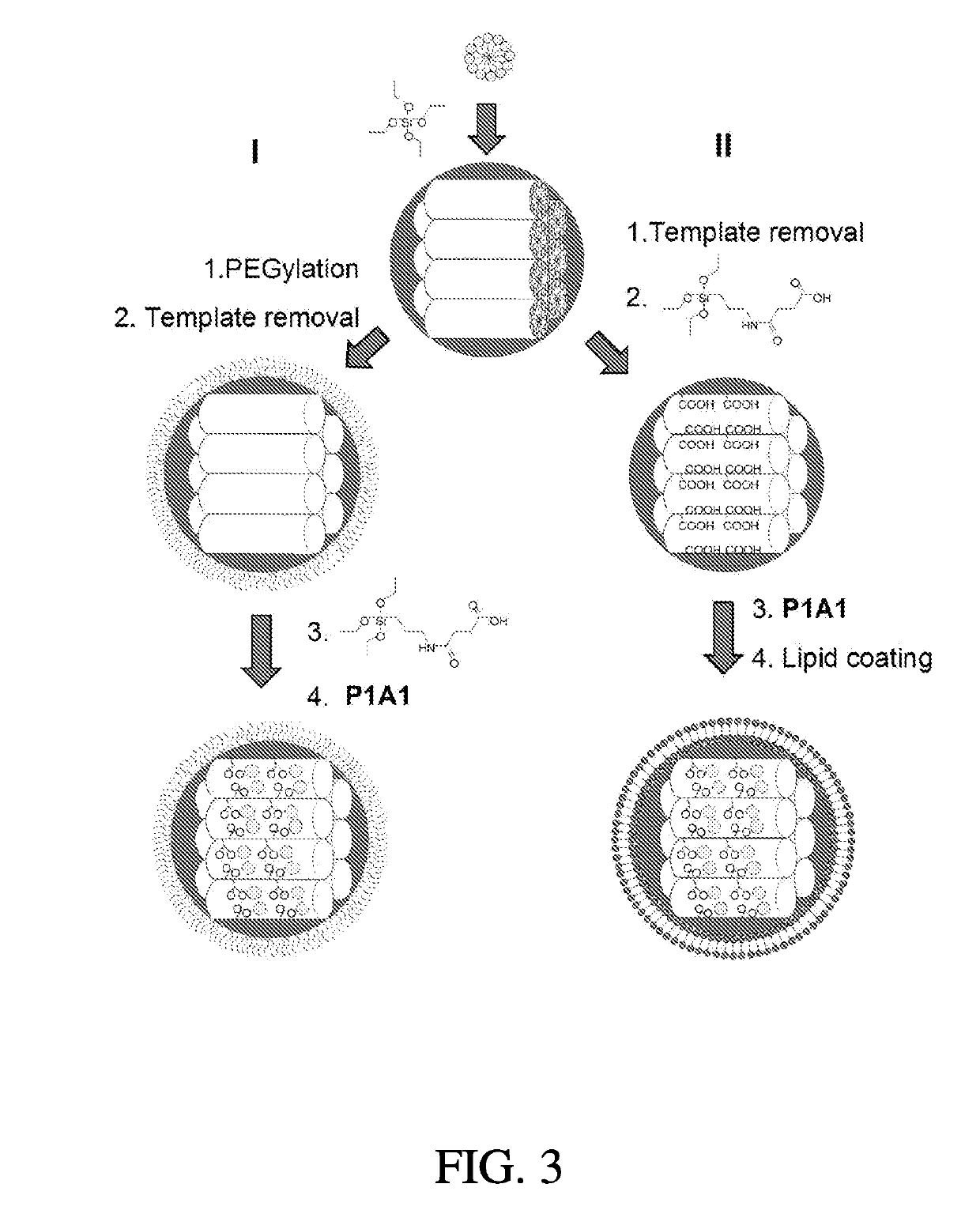
Large-pore mesoporous silica nanoparticles (MSN) were prepared and functionalized to serve as a robust and biocompatible delivery platform for platinum-acridine (PA) anticancer agents. The material showed a high loading capacity for the dicationic, hydrophilic hybrid agent [PtCl(en)(N-[acridin-9-ylaminoethyl]-N-methylropionamidine)] dinitrate salt (P1 Al) and virtually complete retention of payload at neutral pH in a high-chloride buffer. In acidic media mimicking the pH inside the cells' lysosomes, rapid, burst-like release of P1 A1 from the nanoparticles is observed. Coating of the materials in phospholipid bilayers resulted in nanoparticles with greatly improved colloidal stability. The lipid and carboxylate- modified nanoparticles containing 40 wt. % drug caused S phase arrest and inhibited cell proliferation in pancreatic cancer cells at submicromolar concentrations similar to carrier-free P1A1. One feature of the nanoparticle-delivered P1A1 was that the payload did not escape from the acidified lysosomal vesicles into the cytoplasm, but was shuttled to the nuclear membrane and released into the nucleus.
Owner:WAKE FOREST UNIV
N-(3,5-dimethyladamantane-1-yl)-N'-substituted phenylurea compound as well as preparation method and application thereof
ActiveCN107417578AGood anti-Alzheimer's disease activitySmooth responseUrea derivatives preparationNervous disorderAnti alzheimerBiology
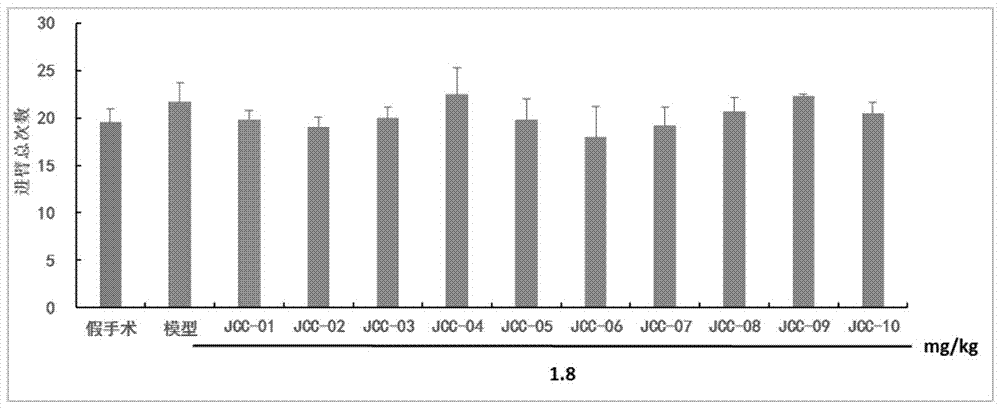
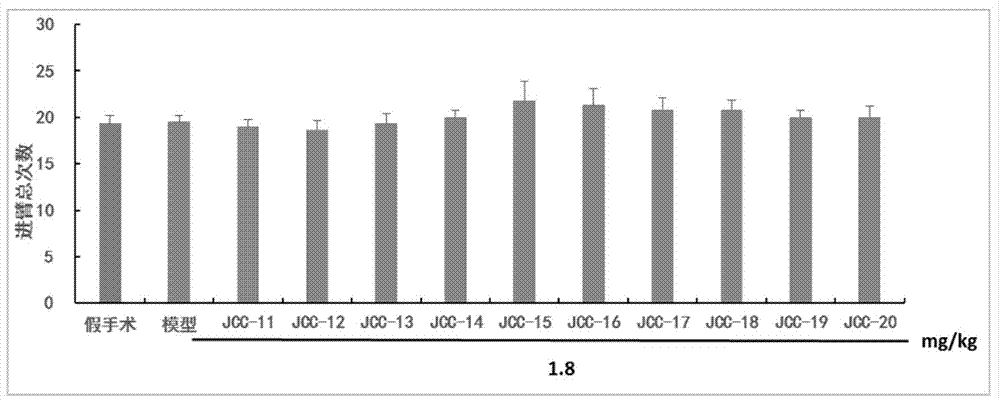
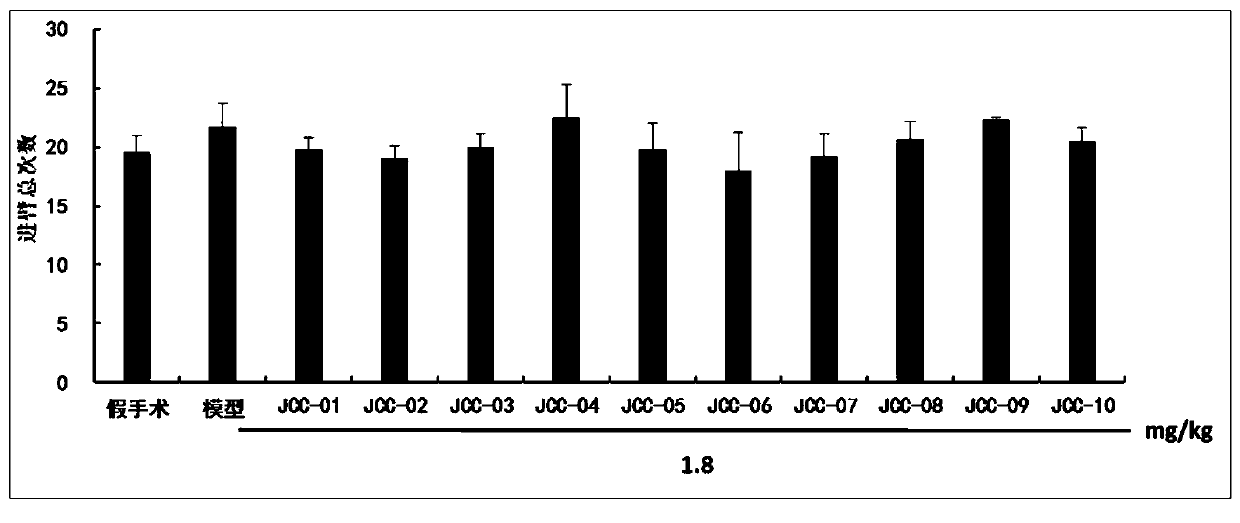
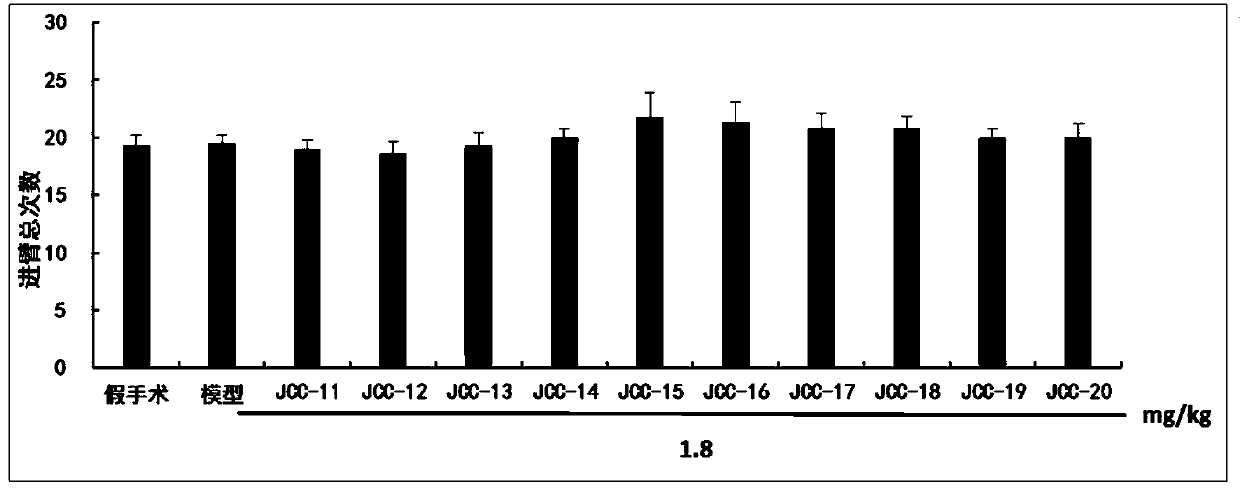
The invention belongs to the technical field of medicinal chemistry, in particular to N-(3,5-dimethyladamantane-1-yl)-N'-substituted phenylurea compounds as well as preparation methods and application thereof. The compounds have the structure shown in the formula I. The compounds are proved that the compounds can improve the image recognition memory, the working and learning memory, the spatial learning and memory ability of model rats and has good anti-alzheimer effects by the new object discrimination experiments, Y-maze experiments, positioning navigation and space exploration experiments in mats. The formula I is shown in the description.
Owner:沈阳海诺威医药科技有限公司
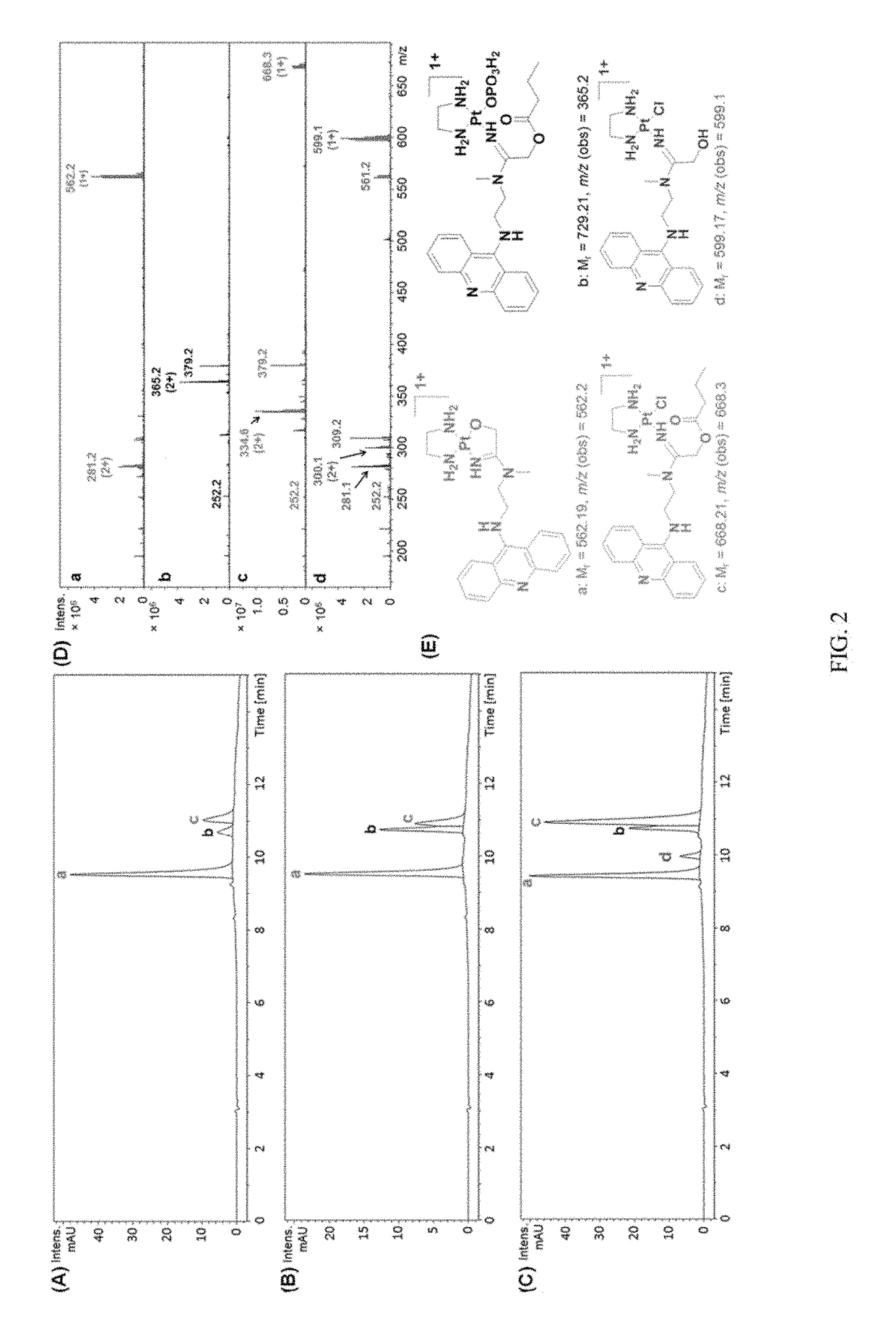
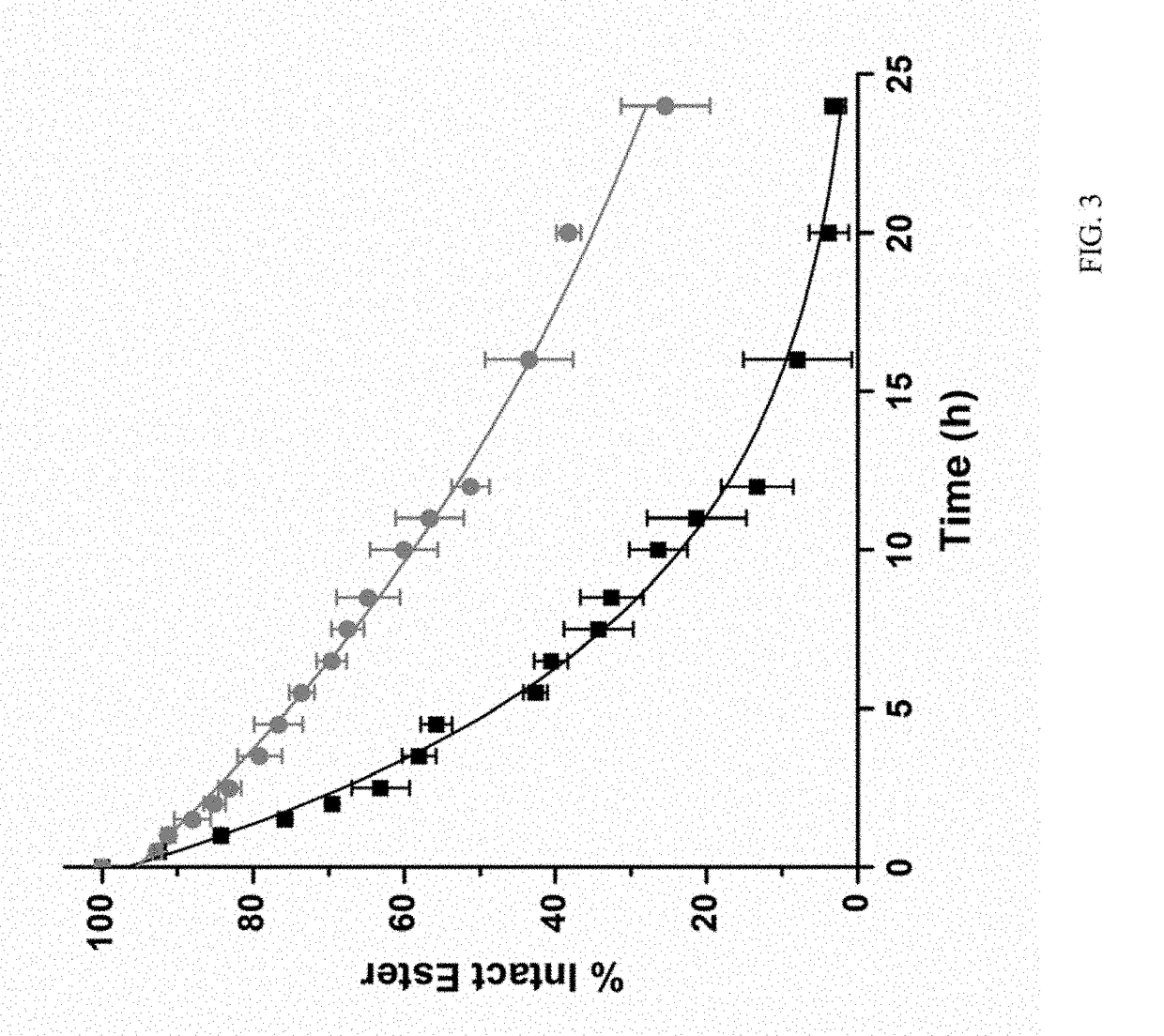
Cleavable conjugates of functionalized platinum-acridine and platinum-benzacridine agents and methods thereof
InactiveUS9765103B2Good pharmacological propertiesPromoting platinum-acridinesOrganic active ingredientsPlatinum organic compoundsCarbamateDual mode
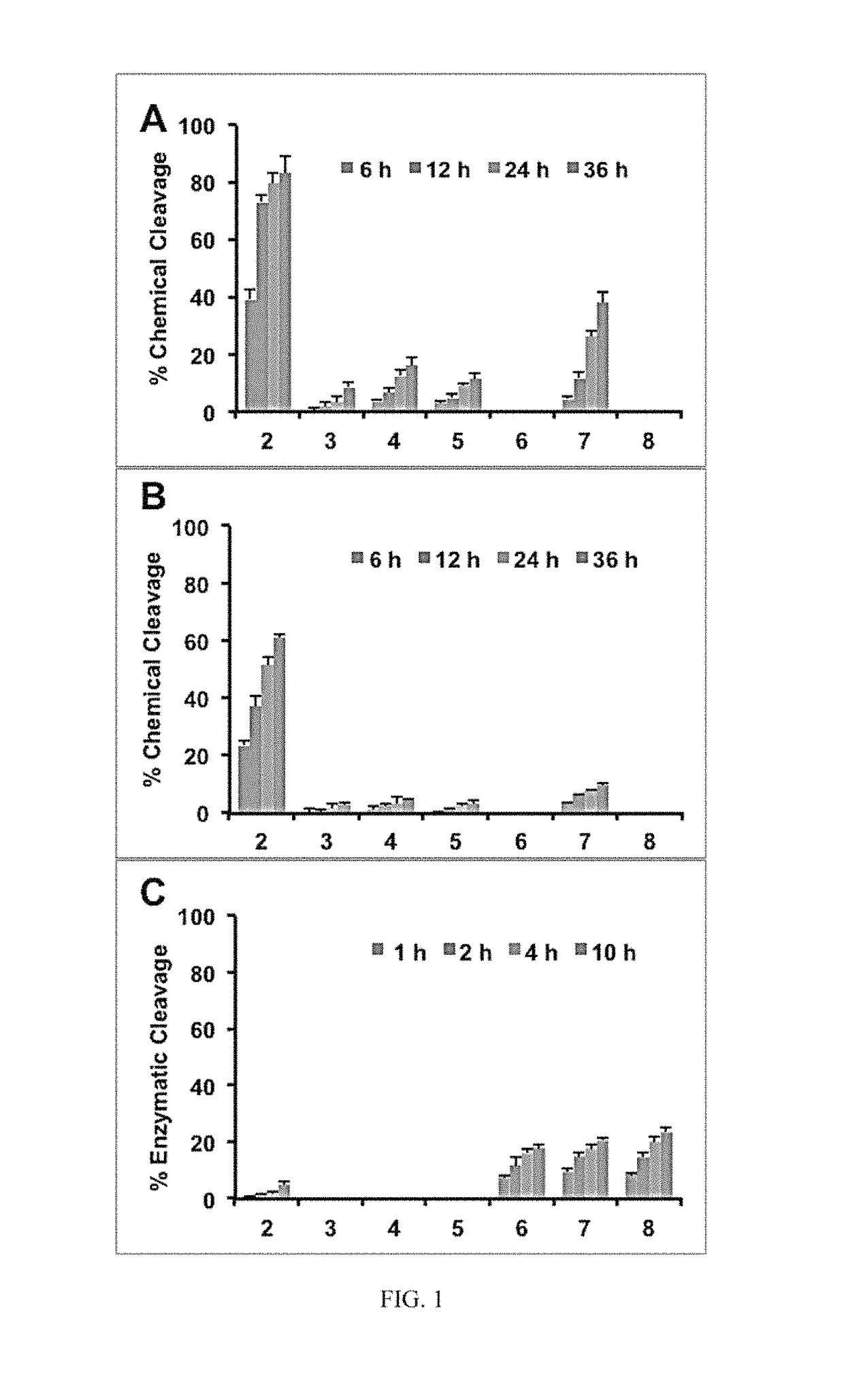
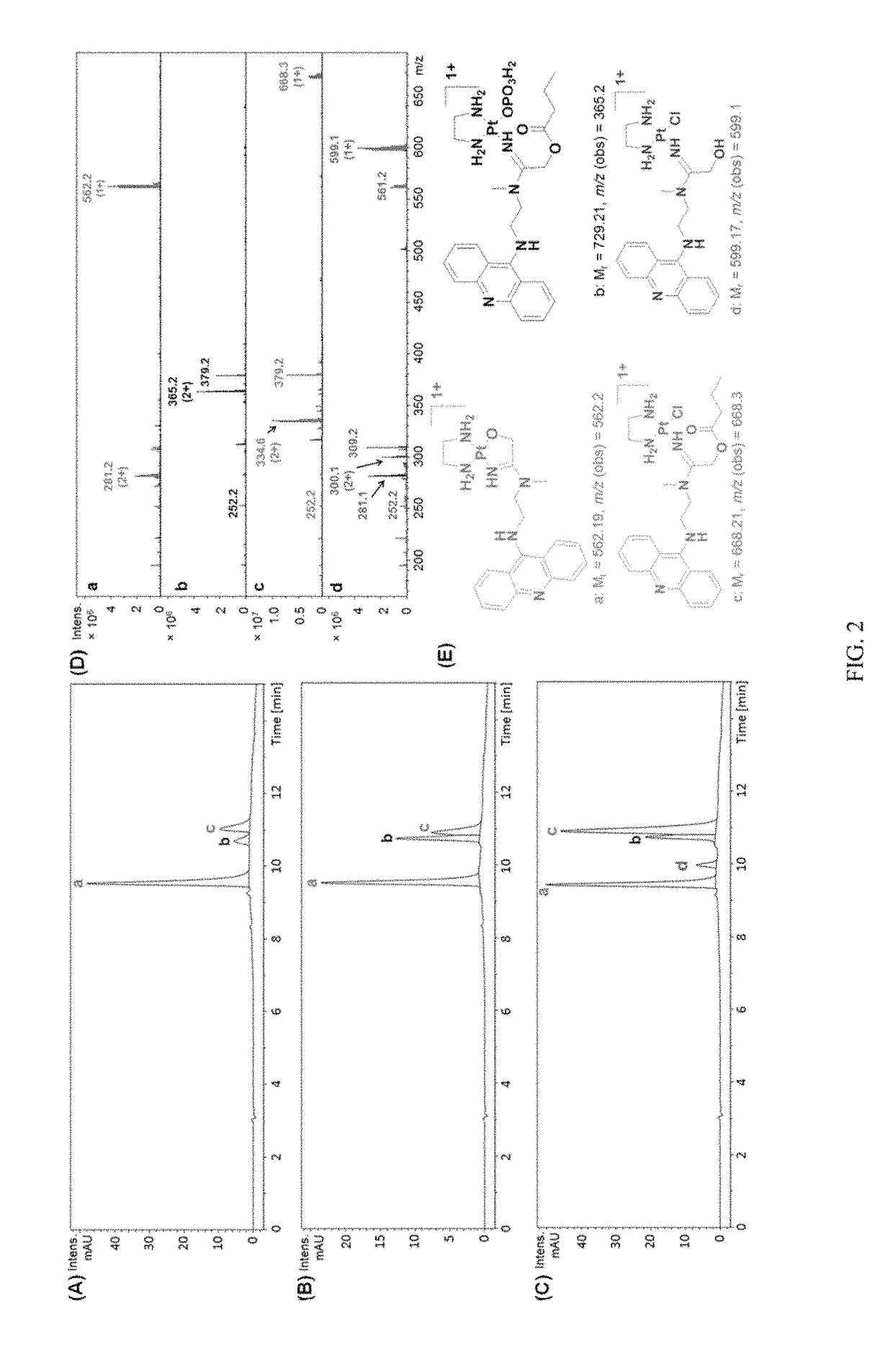
The present invention relates to using a versatile synthetic approach to generate a new class of ester, amido, or carbamate prodrugs of highly potent, but systemically too toxic, platinum-acridine anticancer agents. The new hybrids contain a hydroxyl group, which has been masked with a cleavable lipophilic acyl moiety. Both butanoic (butyric) and bulkier 2-propanepentanoic (valproic) esters were introduced to these compounds. The goal of this design was to improve the drug-like properties of the pharmacophore (e. g., log D) without compromising its DNA-mediated cell kill potential. Two distinct pathways by which the target compounds undergo effective ester hydrolysis, the proposed activating step, have been confirmed: platinum-mediated, self-immolative ester cleavage in a low-chloride environment (LC-ESMS, NMR spectroscopy) and enzymatic cleavage by human carboxylesterase-2 (hCES-2) (LC-ESMS). Several of the new compounds show excellent stability, reduced systemic toxicity, and favorable activation profiles while maintaining submicromolar cytotoxicity in various cancers, such as lung adenocarcinoma cell lines (A549, NCI-H1435). The results suggest that the novel dual-mode prodrug concept may have the potential to hasten the preclinical development of platinum-acridines.
Owner:WAKE FOREST UNIV
Freeze-dried preparation and preparation method and applications thereof
PendingCN110025623AFast disintegrationDisintegration speed is smallCosmetic preparationsPowder deliveryFreeze-dryingTriterpenoid saponin
Owner:李和伟
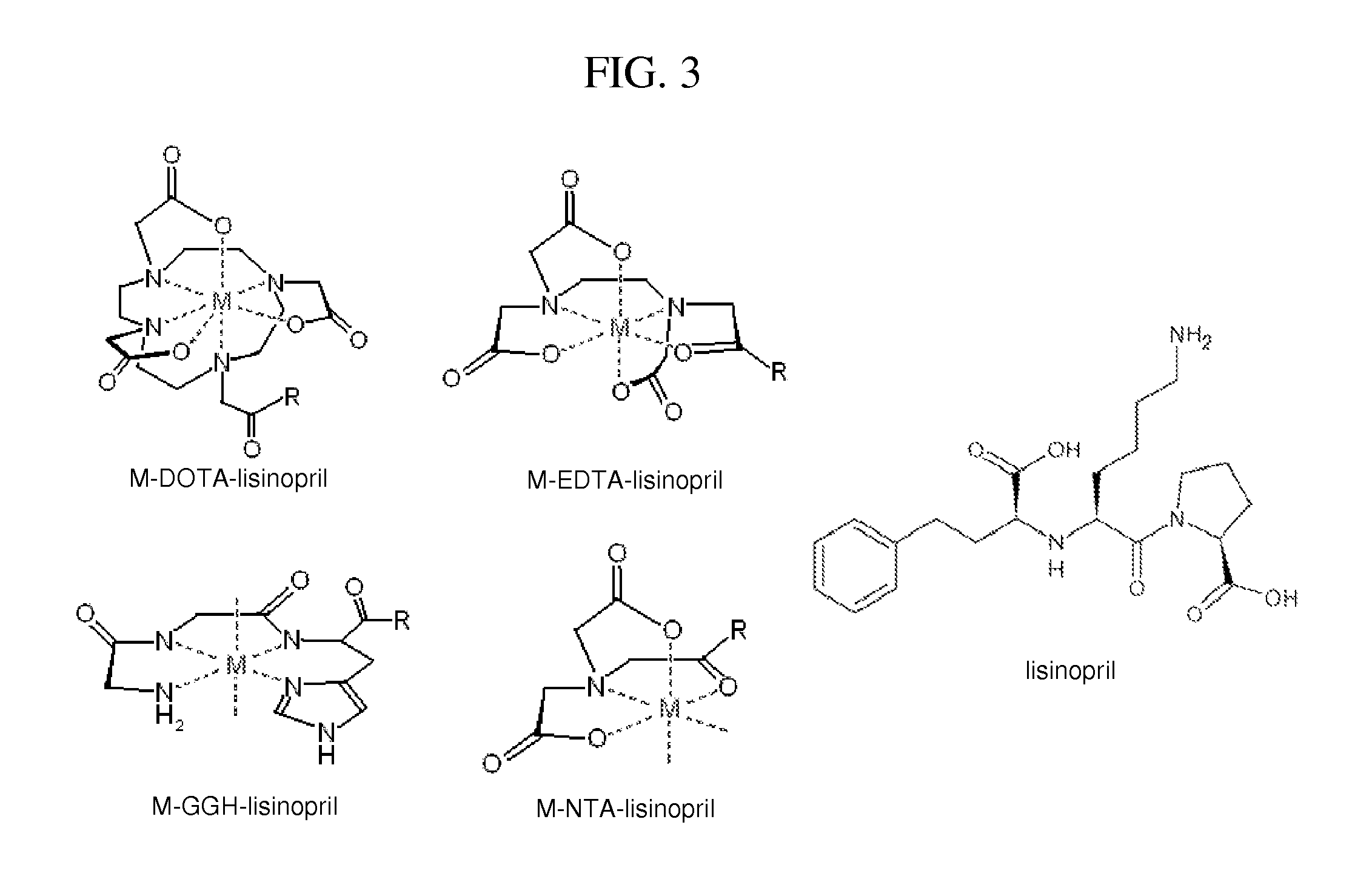
Metallodrugs Having Improved Pharmacological Properties, and Methods of Manufacture and Use Thereof
InactiveUS20140377289A1Good pharmacological propertiesFacilitated reaction kineticsOrganic active ingredientsBiocideLow affinityCatalytic oxidation
It is an object of the present invention to provide improved pharmacological properties to molecules which bind to a target with low affinity (hereinafter referred to as a “ligand moiety”) through linkage of such molecules to a metal binding moiety, thereby generating a combination molecule commonly referred to as a “metallodrug” or “metallotherapeutic.” The metal binding domain of metallodrugs typically catalyzes oxido-reductase chemistry or acts as a Lewis-Acid catalyst, resulting in modification of proteins and nucleic acids that are in close proximity due to binding of the ligand moiety to its target.
Owner:METALLOPHARM
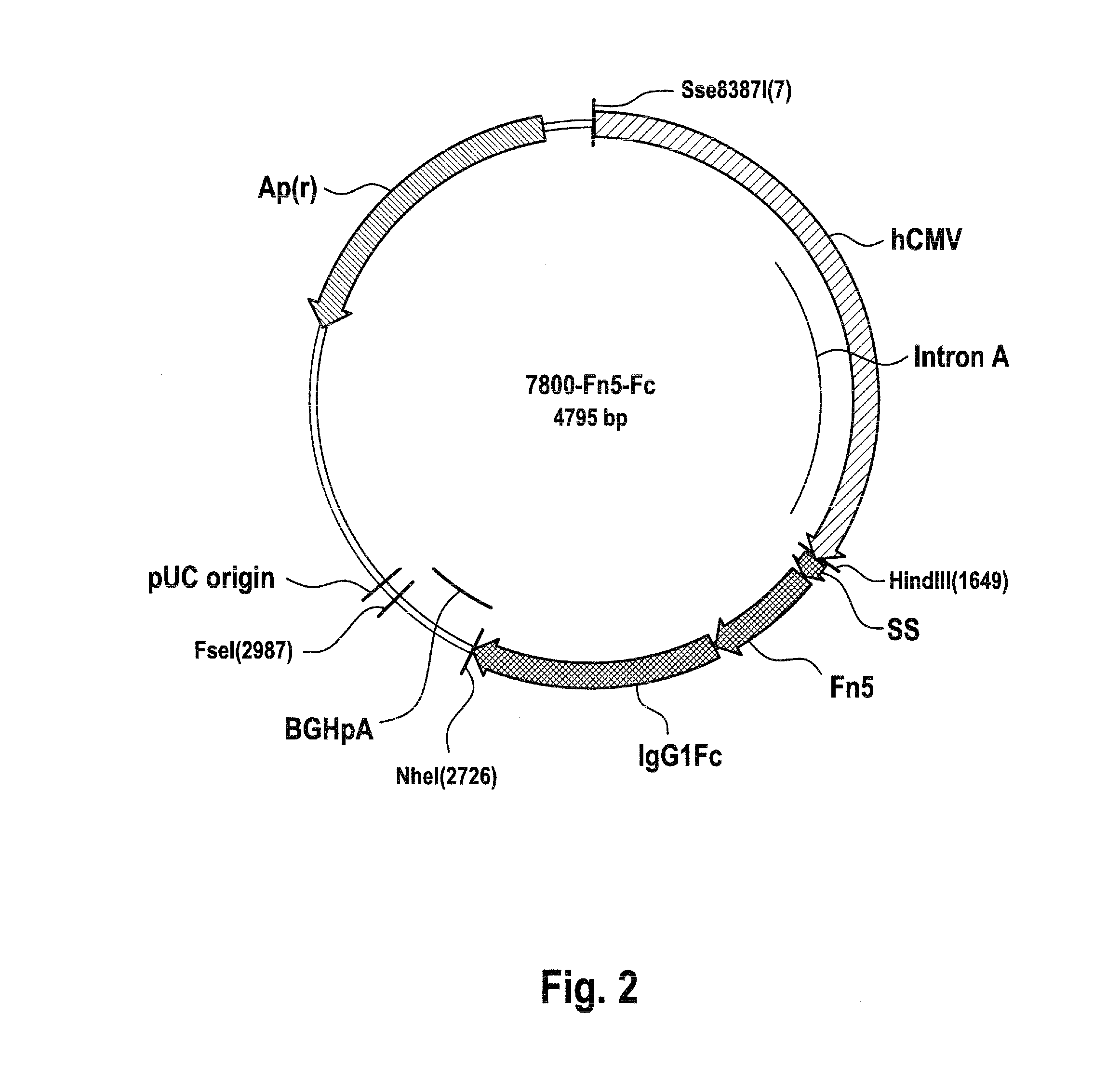
Recombinant fc-fusion protein of the fifth fibronectin type iii domain of dcc
InactiveUS20130336972A1Good pharmacological propertiesHigh affinityAnimal cellsSugar derivativesOncologyColorectal cancer
The present invention relates to DCC-fusion proteins, nucleic acid molecules encoding the DCC-fusion proteins, as well as methods for their production and their use in treatment of cancer such as colorectal cancer, NSCLC and metastatic breast cancer. The present invention also relates to methods of treating cancer such as colorectal cancer, NSCLC and metastatic breast cancer by administering DCC-fusion proteins.
Owner:KLEIN CHRISTIAN +5
Therapeutic use of agonists or antagonists of bradykinin receptor 1 or 2, for modulation collateral blood vessel growth
ActiveUS9492495B2Increasing concentration and half-lifeGood pharmacological propertiesHormone peptidesOrganic active ingredientsBradykinin receptorAgonist
The present invention relates to bradykinin receptor modulators and pharmaceutical compositions thereof for use as a medicament for modulating collateral blood vessel growth of collateral arteries and / or other blood vessels of pre-existing arterial networks. The bradykinin receptor modulators of arteriogenesis are applicable in the treatment and / or prevention of disorders associated with defective blood flow or blood vessel malformation. A preferred aspect of the invention relates to bradykinin receptor agonists for use as a medicament for the prevention of cardiovascular ischemic disease in a patient at risk thereof. Further, the invention relates to a bradykinin receptor agonist for use in a method for treating a cardiovascular ischemic disease in a patient in need thereof, wherein said cardiovascular ischemic disease is a peripheral limb disease.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
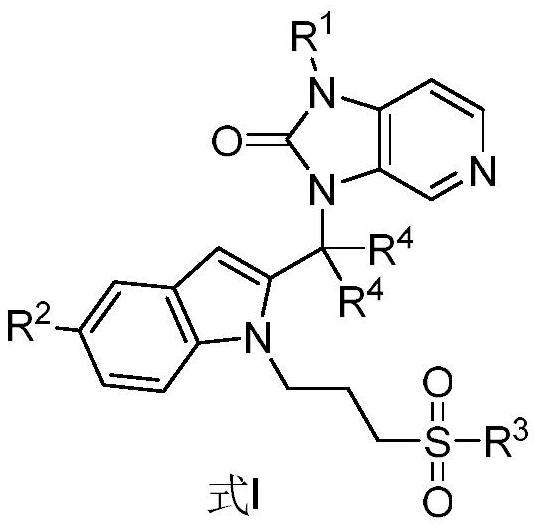
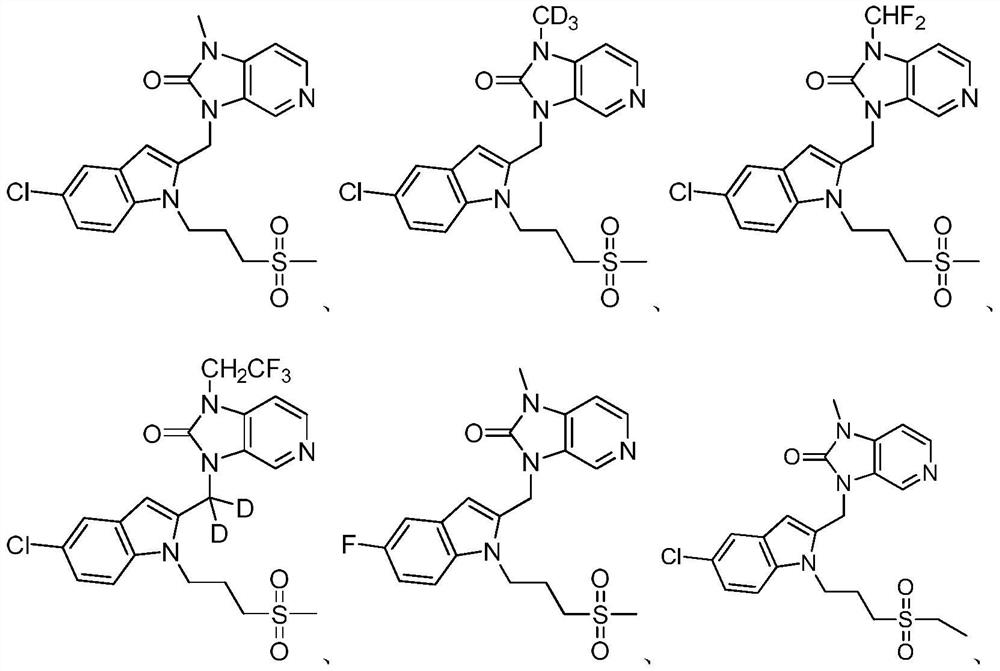
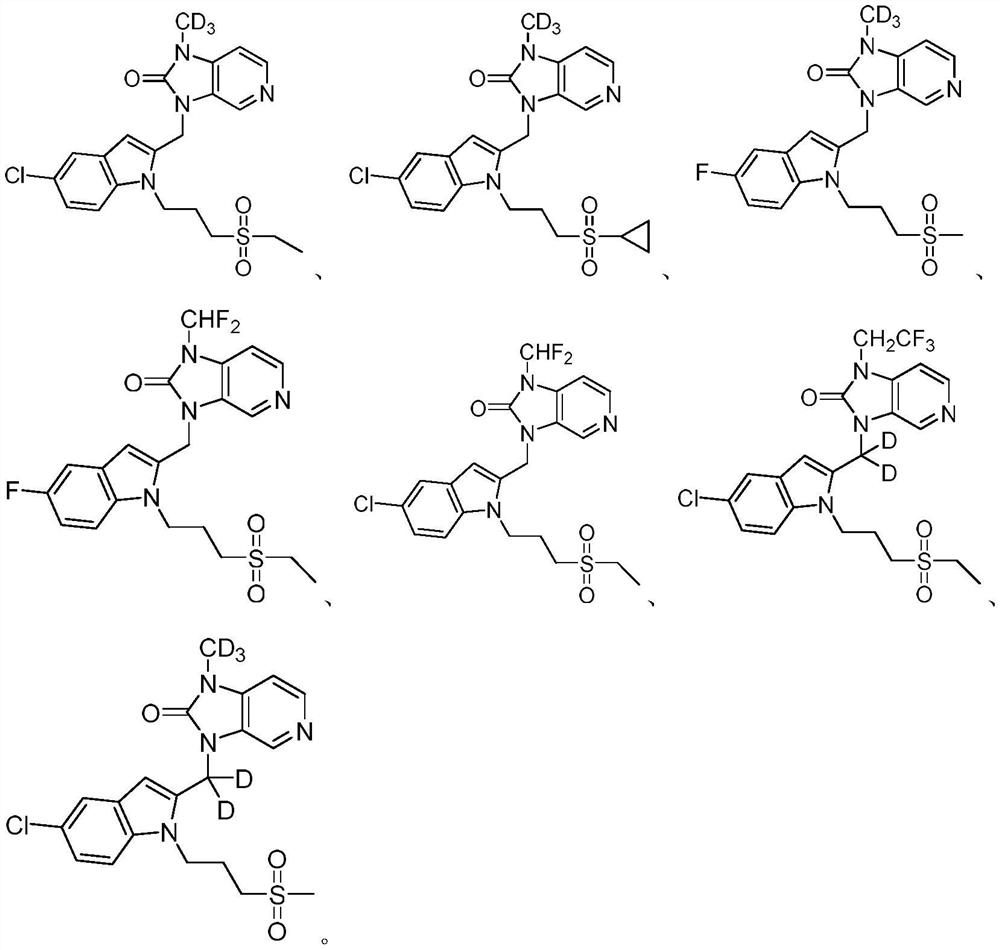
Imidazopyridine derivatives as respiratory syncytial virus antiviral agents
PendingCN114014856AHigh metabolic stabilityPromote oral absorptionOrganic active ingredientsOrganic chemistryRespiratory syncytial virus (RSV)Imidazopyridine
The present invention provides a substituted imidazopyridine derivative, a prodrug, an oxide, a salt, a metal complex or a stereochemical isomer having the structure of Formula I and a pharmaceutical composition containing the same, and a method of using the composition for the treatment of RSV viral infections. The compound provided by the invention has the advantages of high metabolic stability, high solubility, high oral absorbability, good bioavailability, fast detoxification, good antiviral activity, high therapeutic index, low cardiotoxicity and the like.
Owner:ANDIKANG (WUXI) BIOLOGICAL TECH CO LTD
Cleavable Conjugates of Functionalized Platinum-Acridine and Platinum-Benzacridine Agents and Methods Thereof
InactiveUS20170210772A1Good pharmacological propertiesPromoting platinum-acridinesPlatinum organic compoundsRespiratory disorderPre-clinical developmentLow chloride
The present invention relates to using a versatile synthetic approach to generate a new class of ester, amido, or carbamate prodrugs of highly potent, but systemically too toxic, platinum-acridine anticancer agents. The new hybrids contain a hydroxyl group, which has been masked with a cleavable lipophilic acyl moiety. Both butanoic (butyric) and bulkier 2-propanepentanoic (valproic) esters were introduced to these compounds. The goal of this design was to improve the drug-like properties of the pharmacophore (e.g., log D) without compromising its DNA-mediated cell kill potential. Two distinct pathways by which the target compounds undergo effective ester hydrolysis, the proposed activating step, have been confirmed: platinum-mediated, self-immolative ester cleavage in a low-chloride environment (LC-ESMS, NMR spectroscopy) and enzymatic cleavage by human carboxylesterase-2 (hCES-2) (LC-ESMS). Several of the new compounds show excellent stability, reduced systemic toxicity, and favorable activation profiles while maintaining submicromolar cytotoxicity in various cancers, such as lung adenocarcinoma cell lines (A549, NCI-H1435). The results suggest that the novel dual-mode prodrug concept may have the potential to hasten the preclinical development of platinum-acridines.
Owner:WAKE FOREST UNIV
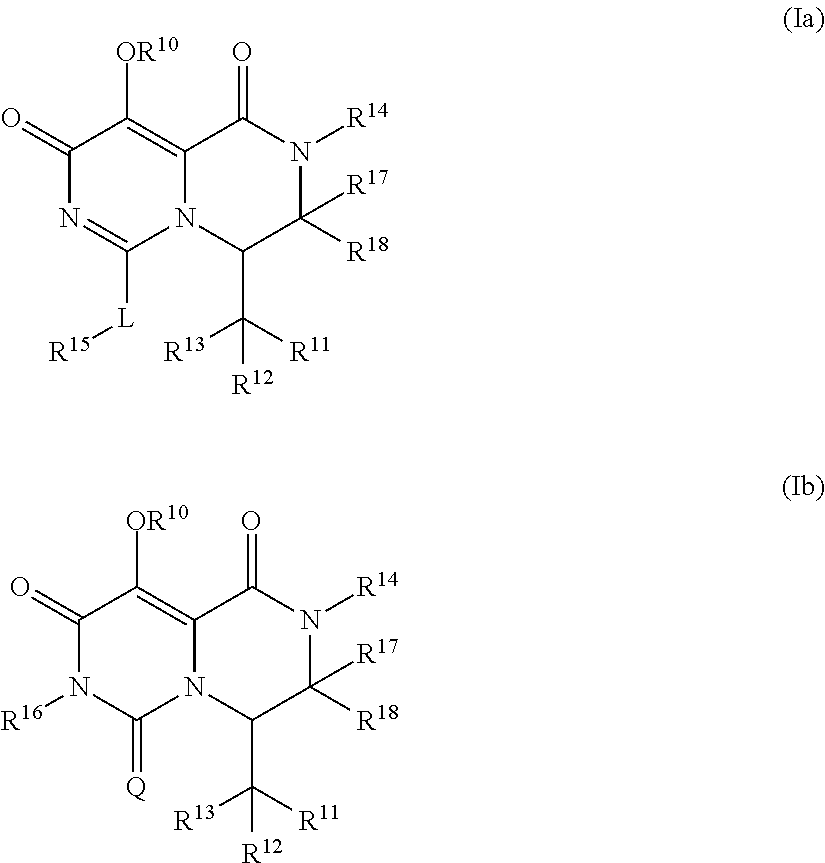
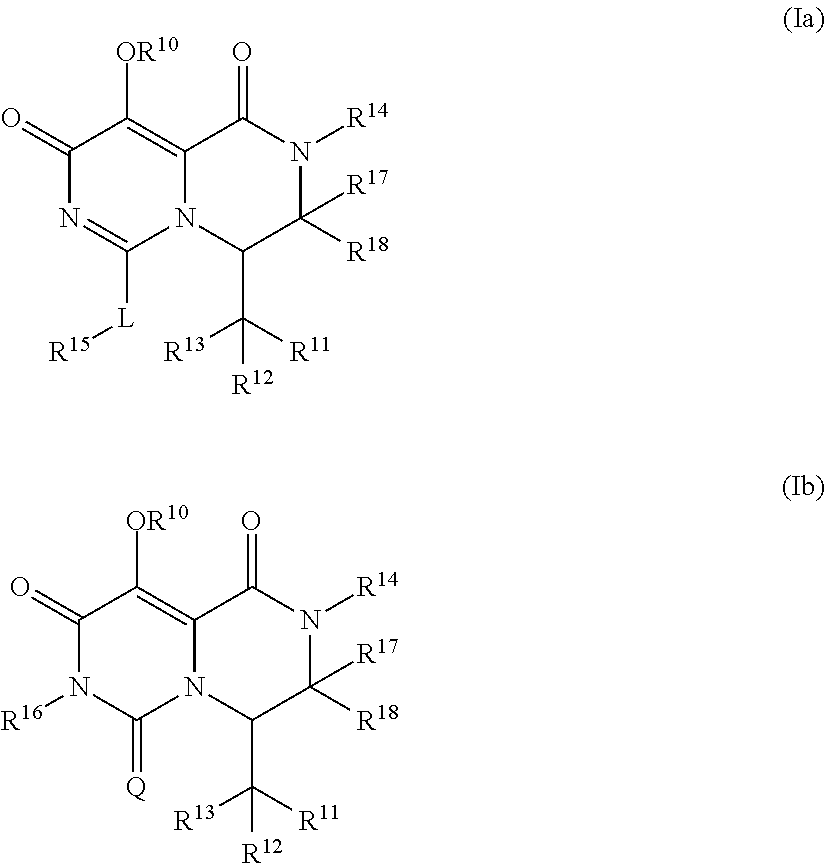
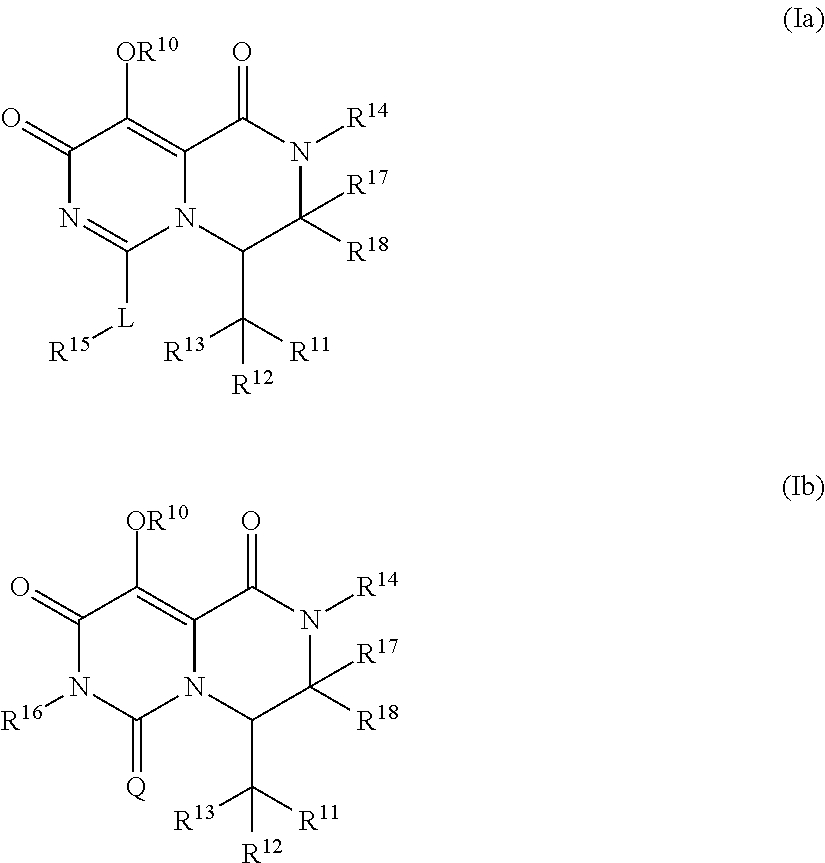
Pyrimidone derivatives and their use in the treatment, amelioration or prevention of a viral disease
InactiveUS20170267680A1Good pharmacological propertiesImprove propertiesOrganic chemistryAntiviralsViral diseaseDiastereomer
The present invention relates to compounds having the general formula (Ia) or (Ib), optionally in the form of a pharmaceutically acceptable salt, solvate, polymorph, codrug, cocrystal, prodrug, tautomer, racemate, enantiomer, or diastereomer or mixture thereof,which are useful in treating, ameloriating or preventing a viral disease, in particular influenza.
Owner:F HOFFMANN LA ROCHE & CO AG
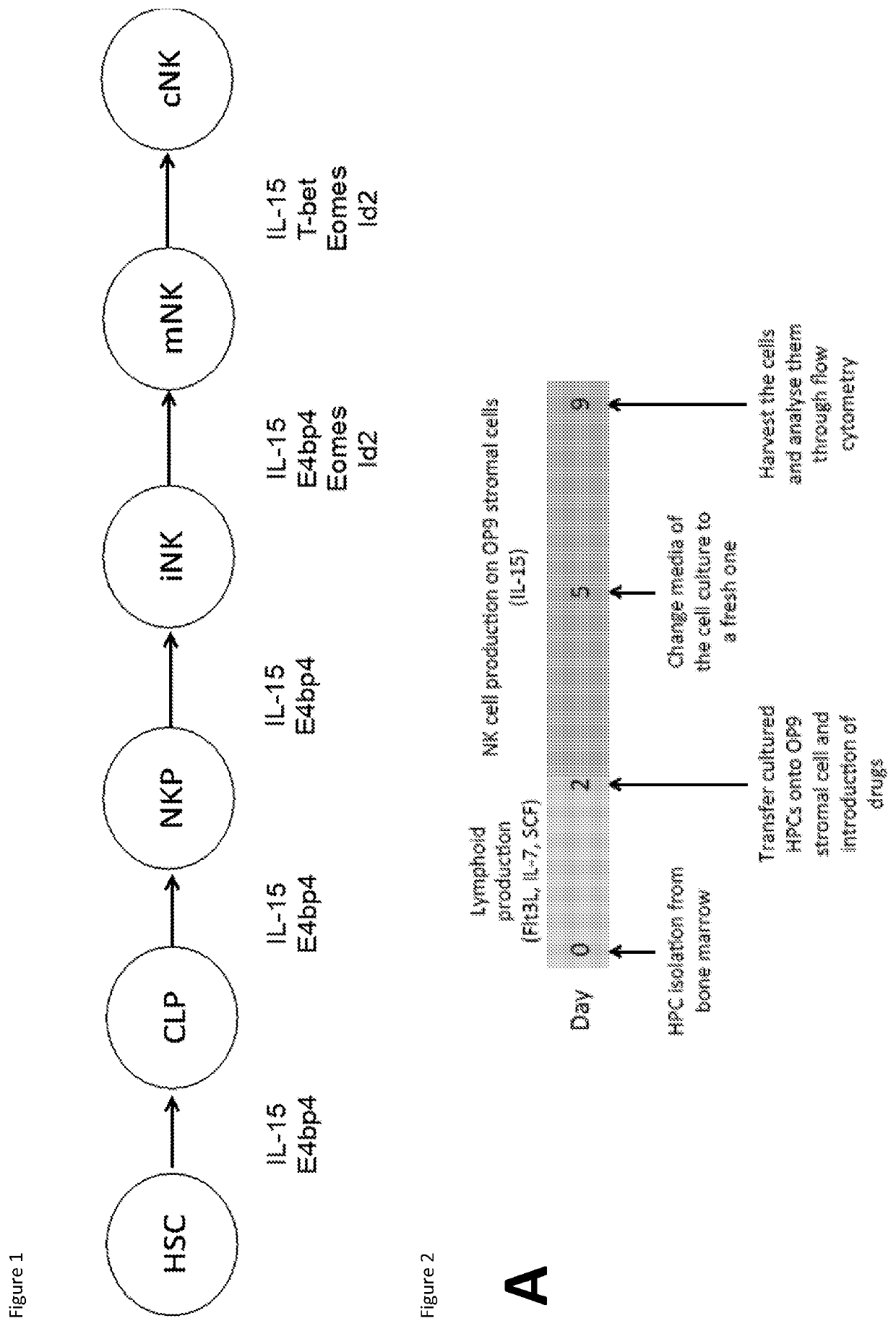
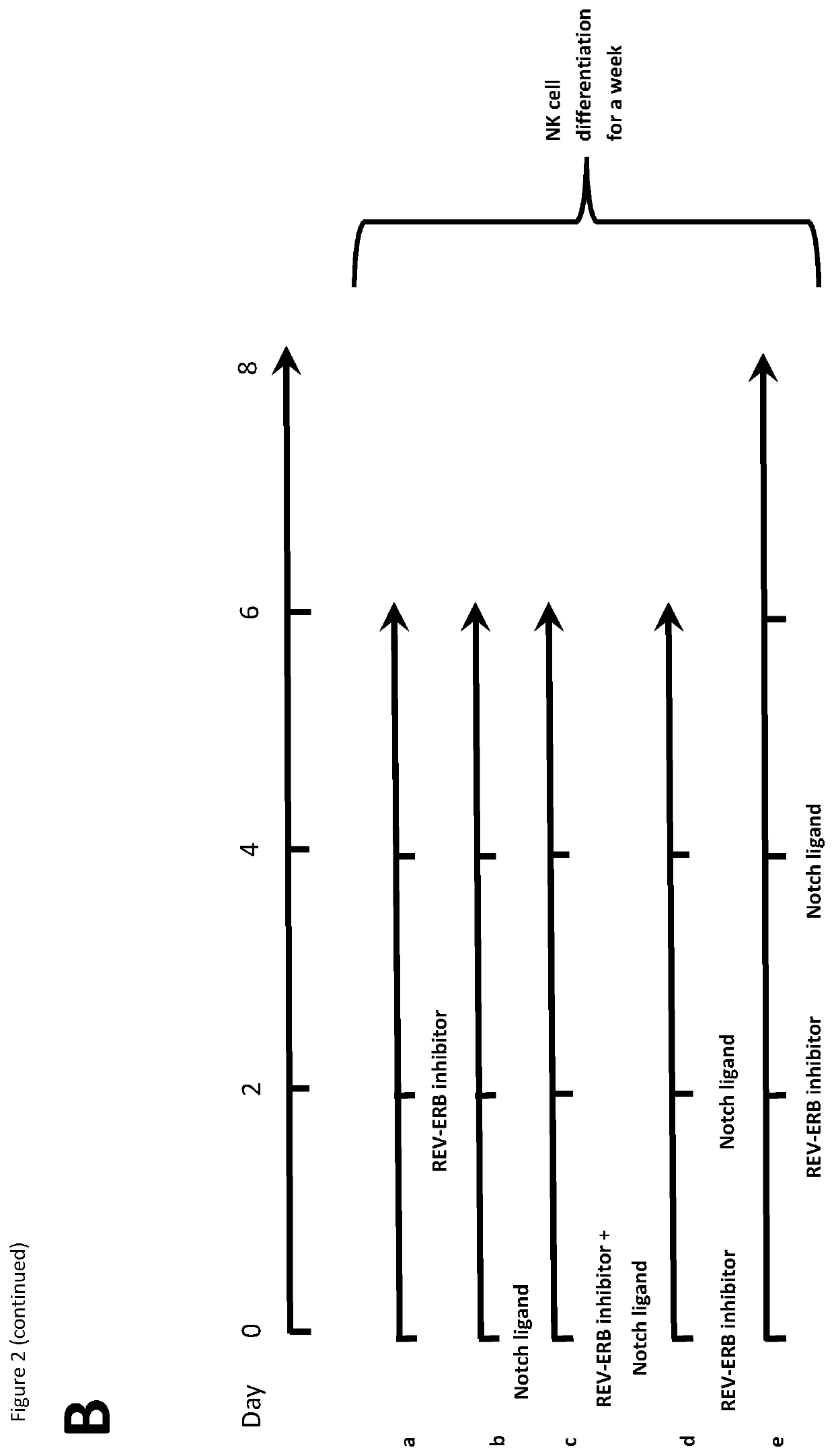
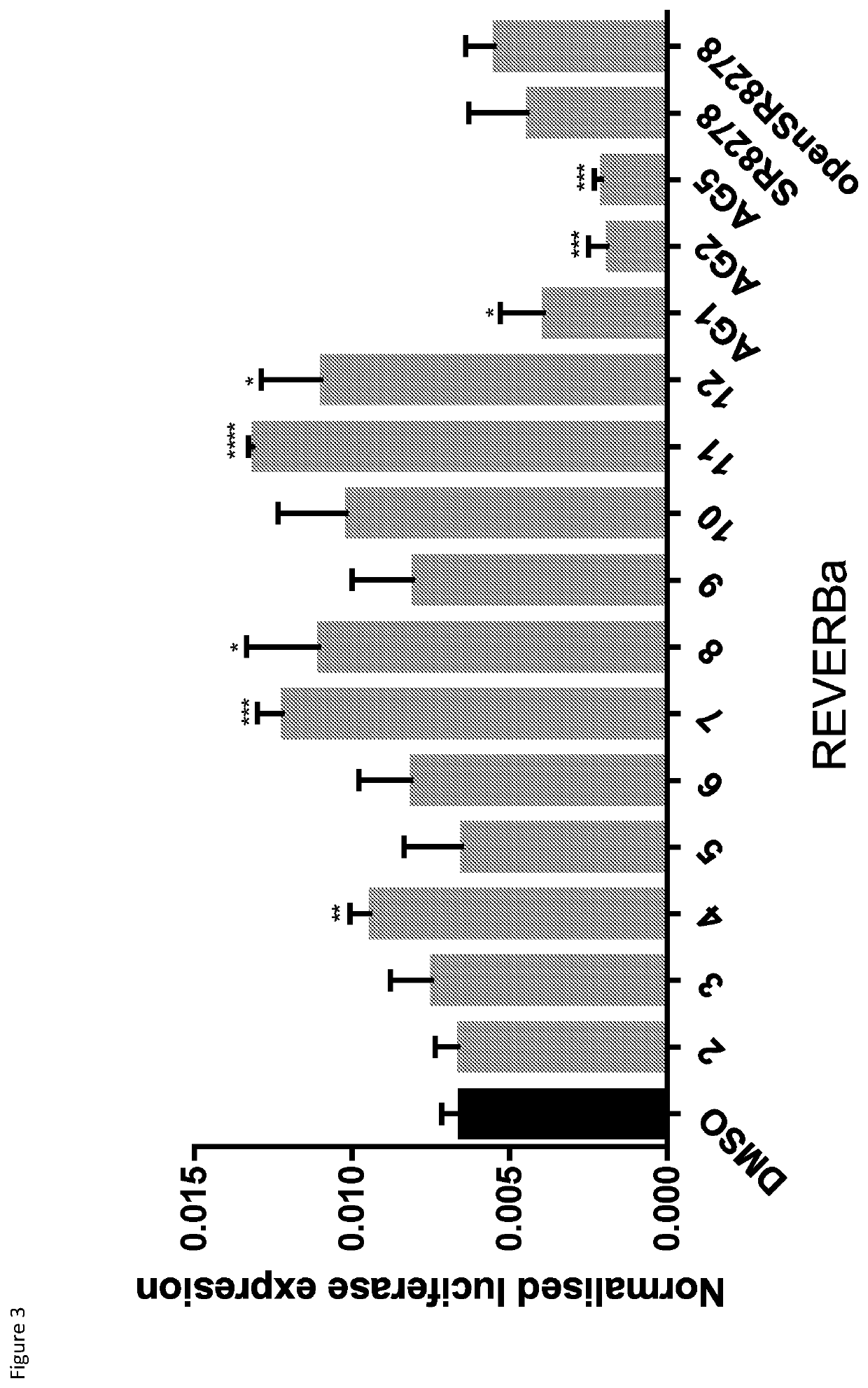
Natural killer cells
PendingUS20220073880A1Avoid actionIncrease productionOrganic chemistryMammal material medical ingredientsNatural Killer Cell Inhibitory ReceptorsBiochemistry
This invention relates to Natural Killer (NK) cell populations, to methods of producing the same and therapeutic applications thereof. More specifically, the invention relates to the expansion of IMK cells by increasing the expression of specific transcription factors associated with NK cell production.
Owner:IMPERIAL INNOVATIONS LTD
1,3-dihydro-1,4-benzodiazepine-2-thiones for the treatment of CNS related diseases
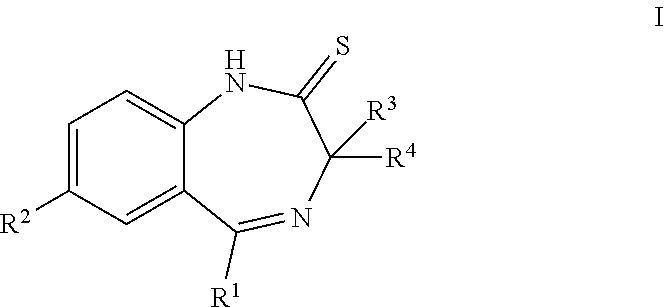
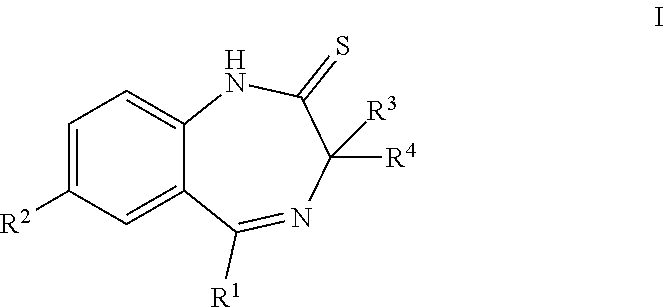
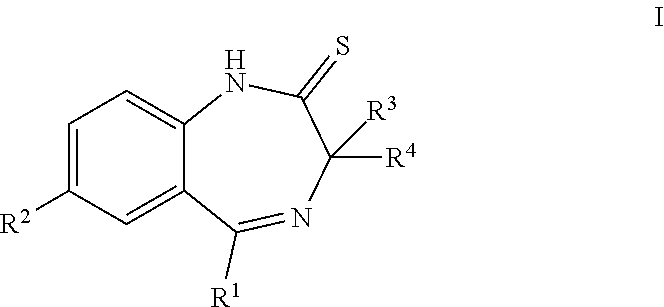
ActiveUS20190256478A1High activityGood pharmacological propertiesNervous disorderOrganic chemistryBenzodiazepineBenzene
The present invention provides compounds that are muscarinic M1 receptor positive allosteric modulators (PAM) and useful in the treatment of diseases, mediated by the muscarinic M1 receptor, such as Alzheimer's disease, cognitive impairment, schizophrenia, pain or sleep disorders, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances.
Owner:F HOFFMANN LA ROCHE & CO AG
MiR-181a-5p nanocomposite for treating oral cancer as well as preparation method and application of miR-181a-5p nanocomposite
PendingCN114796271AImprove targetingImprove efficiencyOrganic active ingredientsInorganic active ingredientsCancer cellMalignancy
The invention provides a miR-181a-5p nano compound for treating oral cancer as well as a preparation method and application of the miR-181a-5p nano compound. The polyethyleneimine modified silver nanoparticles are selected, a culture medium without double antibodies is generally needed during cell transfection, and nano-silver has a certain bacteriostatic effect and can effectively reduce the risk that cells are polluted during transfection, so that the operation is simpler, more convenient, safer and more effective when the nano-carrier is used; and the experiment efficiency can be improved. Besides, the polyethyleneimine modified nano-silver compound has certain pH responsiveness, the targeting of nano-drugs to tumor sites and the release efficiency of gene sequences can be remarkably improved, and research on in-vivo and in-vitro anti-tumor mechanisms is more convenient. The nano-carrier is used for delivering miR-181a-5p with an oral cancer inhibition function, and the expression quantity of miR-181a-5p in cells can be improved while the serum stability and RNA enzyme stability of mimics of miR-181a-5p can be remarkably improved, so that malignant behaviors such as proliferation, migration and invasion of oral cancer cells are effectively inhibited.
Owner:SHANXI MEDICAL UNIV
Metallodrugs having improved pharmacological properties, and methods of manufacture and use thereof
InactiveUS8859492B2Good pharmacological propertiesFacilitated reaction kineticsOrganic active ingredientsBiocideLow affinityCatalytic oxidation
It is an object of the present invention to provide improved pharmacological properties to molecules which bind to a target with low affinity (hereinafter referred to as a “ligand moiety”) through linkage of such molecules to a metal binding moiety, thereby generating a combination molecule commonly referred to as a “metallodrug” or “metallotherapeutic.” The metal binding domain of metallodrugs typically catalyzes oxido-reductase chemistry or acts as a Lewis-Acid catalyst, resulting in modification of proteins and nucleic acids that are in close proximity due to binding of the ligand moiety to its target.
Owner:METALLOPHARM
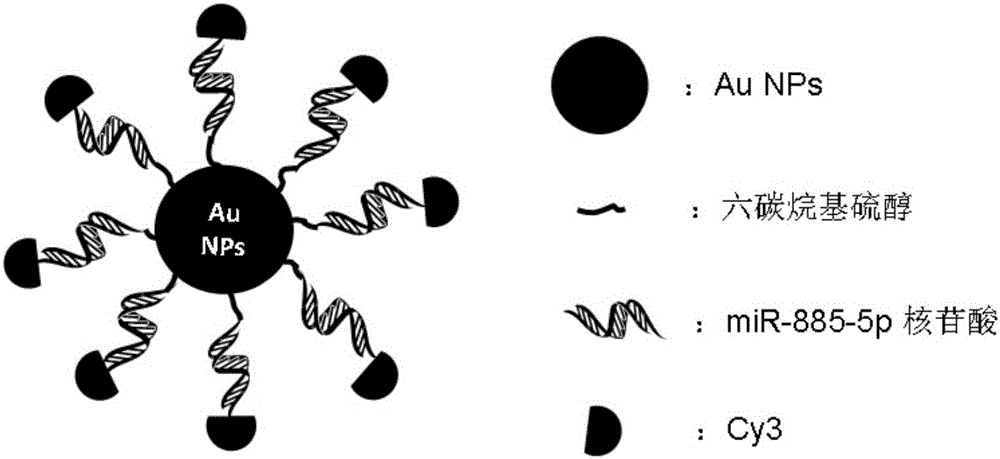
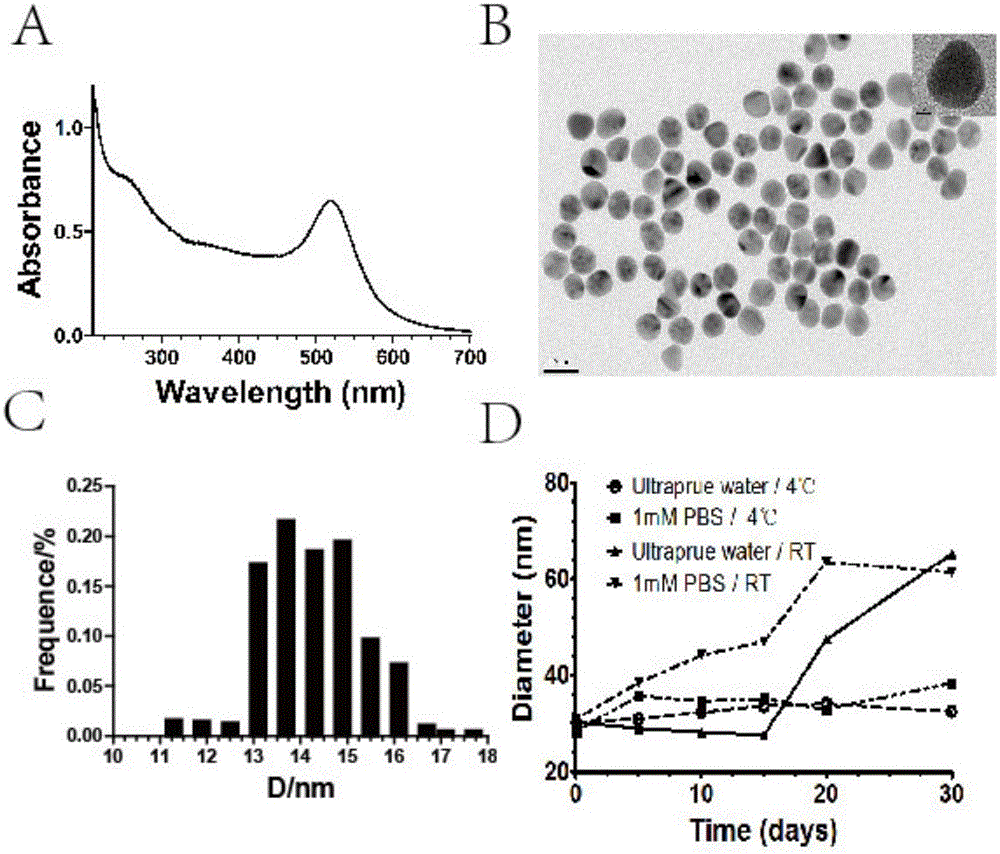
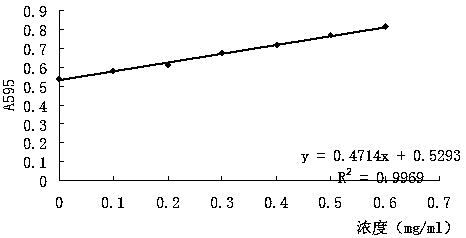
Nano gold mir-885-5p conjugate and its preparation method and application
ActiveCN103800917BGood pharmacological propertiesImprove stabilityOrganic active ingredientsGenetic material ingredientsNucleotideGold particles
The invention discloses a nano-gold miR-885-5p conjugate as well as a preparation method and an application thereof. The conjugate comprises nano-gold particles and double-stranded nucleotide, wherein the double-stranded nucleotide is double-stranded miR-885-5p of which the double strands both have 28 bases, or is double-stranded nucleotide which has a sequence homology of 95%-100% with the double-stranded miR-885-5p and has the same coding function as the double-stranded miR-885-5p, wherein the double-stranded nucleotide comprises two stem-loop structures, and a 5'-terminal of each antisense strand contains hexacarbon alkyl mercaptan and is coupled with the nano-gold particles. The nano-gold miR-885-5p conjugate has a remarkable growth inhibition effect on both liver cancer cells in vitro and liver cancer nude mouse transplanted tumors in vivo, and has a beneficial effect in the application for treating the liver cancer.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Sarsasapogenin derivative and preparation method as well as application thereof
InactiveCN108117580AGood pharmacological propertiesSmooth responseOrganic active ingredientsNervous disorderNervous systemMolecular level
The invention belongs to the technical field of medicinal chemistry and particularly relates to a sarsasapogenin derivative and a preparation method as well as application thereof. The compound has structures shown in formulae I, II, III, IV, V, VI and VII as shown in the specification. Through the proof of a molecular level experiment, a cellular level experiment, and an APP transgenic mouse place navigation and space exploration experiment, the compound can improve the spatial learning memory capability of model mice, and has a good effect of treating degenerative diseases of nervous systems.
Owner:SHENYANG PHARMA UNIVERSITY
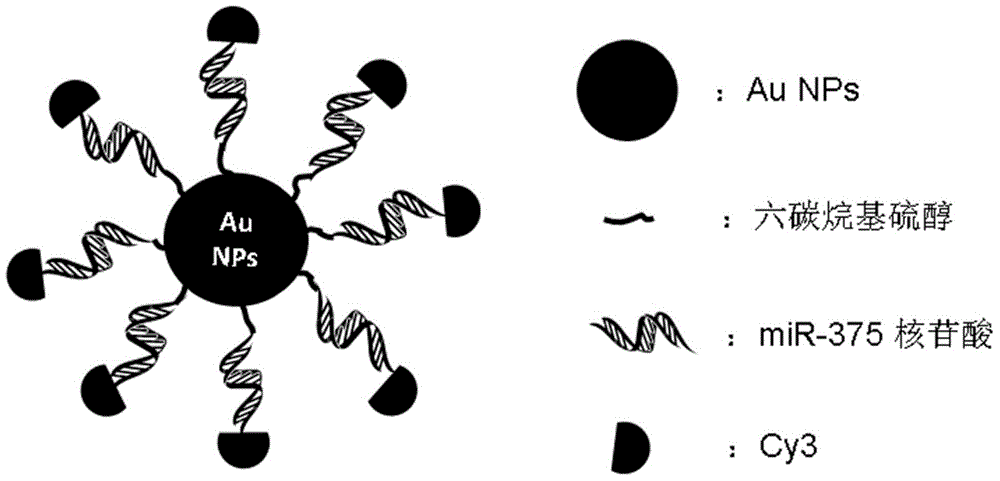
Nano-gold mir-375 conjugates, preparation method and application thereof
ActiveCN103768617BGood pharmacological propertiesImprove stabilityOrganic active ingredientsGenetic material ingredientsNucleotideGold particles
The invention discloses a nano-gold miR-375 conjugate, and a preparation method and application thereof. The conjugate comprises nano-gold particles and double-strand nucleotide, wherein two strands of the double-strand nucleotide are double-strand miR-375 with 28 basic groups, or double-strand nucleotide with homology of 95-100 percent and same coding function of the double-strand miR-375 sequence; the double-strand oligonucleotide has two stem-loop structures, and the 5' terminal of an antisense strand has C6 alkyl mercaptan, and is coupled with the nano-gold particles. The nano-gold miR-375 conjugate has a remarkably growth inhibiting effect on in-vitro liver cancer cells and in-vivo transplantation tumor in nude mice with liver cancer, has beneficial effects in liver cancer treatment and application, and can be potentially applied to treatment of tumors of other digestive systems.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Ophthalmic pharmaceutical composition containing a carbonic anhydrase inhibitor and method for the preparation thereof
InactiveUS20150080385A1Sufficient self-lifeBioavailableSenses disorderInorganic non-active ingredientsOcular hypertensionPharmaceutical formulation
The present invention relates to a stable pharmaceutical formulation for topical administration containing a therapeutically effective quantity of Brinzolamide or ophthalmologic acceptable salts thereof and an effective quantity of a surfactant such as poloxamer, to be used for the treatment of ocular hypertension and glaucoma.
Owner:PHARMATHEN
Cationic elaioplast and its adenovirus composition, its preparing method and use
ActiveCN101045037BSimple compositionImprove biological effectGenetic material ingredientsPharmaceutical non-active ingredientsCholesterolElaioplast
A cationic liposome is proportionally prepared from DOPE, DOTMA and cholesterol and can be used to encapsulate the object (adenovirus carrier) to obtain a recombinant human endostatin adenovirus-cationic liposome composition carrying the active antineoplastic genes for suppressing the growth of tumor. Its preparing process is also disclosed.
Owner:SICHUAN UNIV
N-(3,5-dimethyladamantan-1-yl)-n'-substituted phenylurea compounds and their preparation methods and uses
ActiveCN107417578BSmooth responseIncrease independenceUrea derivatives preparationNervous disorderPhysical chemistryCombinatorial chemistry
The invention belongs to the technical field of medicinal chemistry, in particular to N-(3,5-dimethyladamantane-1-yl)-N'-substituted phenylurea compounds as well as preparation methods and application thereof. The compounds have the structure shown in the formula I. The compounds are proved that the compounds can improve the image recognition memory, the working and learning memory, the spatial learning and memory ability of model rats and has good anti-alzheimer effects by the new object discrimination experiments, Y-maze experiments, positioning navigation and space exploration experiments in mats. The formula I is shown in the description.
Owner:沈阳海诺威医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors](https://images-eureka.patsnap.com/patent_img/f4f3d988-e07f-4a18-972e-f0c8d69c4fb5/US20120302549A1-20121129-C00001.png)
![Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors](https://images-eureka.patsnap.com/patent_img/f4f3d988-e07f-4a18-972e-f0c8d69c4fb5/US20120302549A1-20121129-C00002.png)
![Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as bace1 and/or bace2 inhibitors](https://images-eureka.patsnap.com/patent_img/f4f3d988-e07f-4a18-972e-f0c8d69c4fb5/US20120302549A1-20121129-C00003.png)
![N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors](https://images-eureka.patsnap.com/patent_img/55ad4707-d05d-4b47-940d-c2be54d94c06/US20130072478A1-20130321-C00001.png)
![N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors](https://images-eureka.patsnap.com/patent_img/55ad4707-d05d-4b47-940d-c2be54d94c06/US20130072478A1-20130321-C00002.png)
![N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors N-(3-(2-amino-6,6-difluoro-4,4a,5,6,7,7a-hexahydro-cyclopenta[e][1,3]oxazin-4-yl)-phenyl-amides as BACE1 inhibitors](https://images-eureka.patsnap.com/patent_img/55ad4707-d05d-4b47-940d-c2be54d94c06/US20130072478A1-20130321-C00003.png)