Freeze-dried preparation and preparation method and applications thereof
A freeze-dried preparation and freeze-drying technology, applied in biochemical equipment and methods, freeze-dried delivery, skin care preparations, etc., can solve the problems of limited drug loading, complex structure, disintegration and poor dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
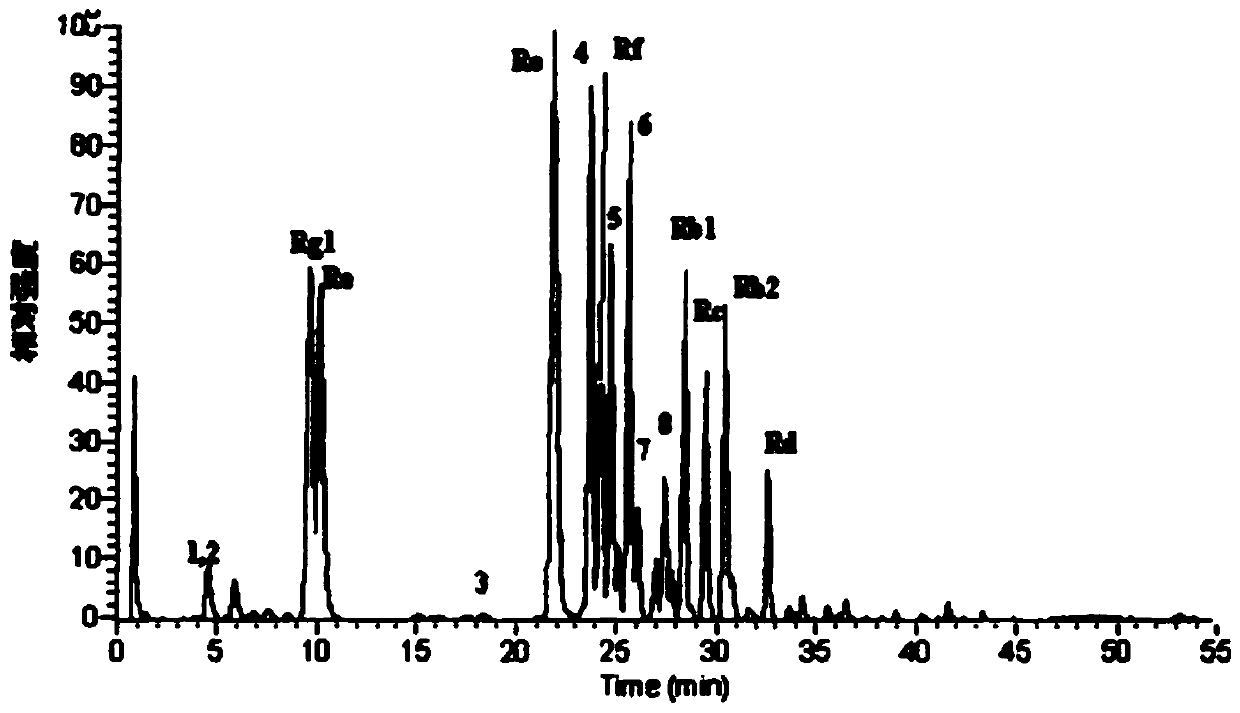
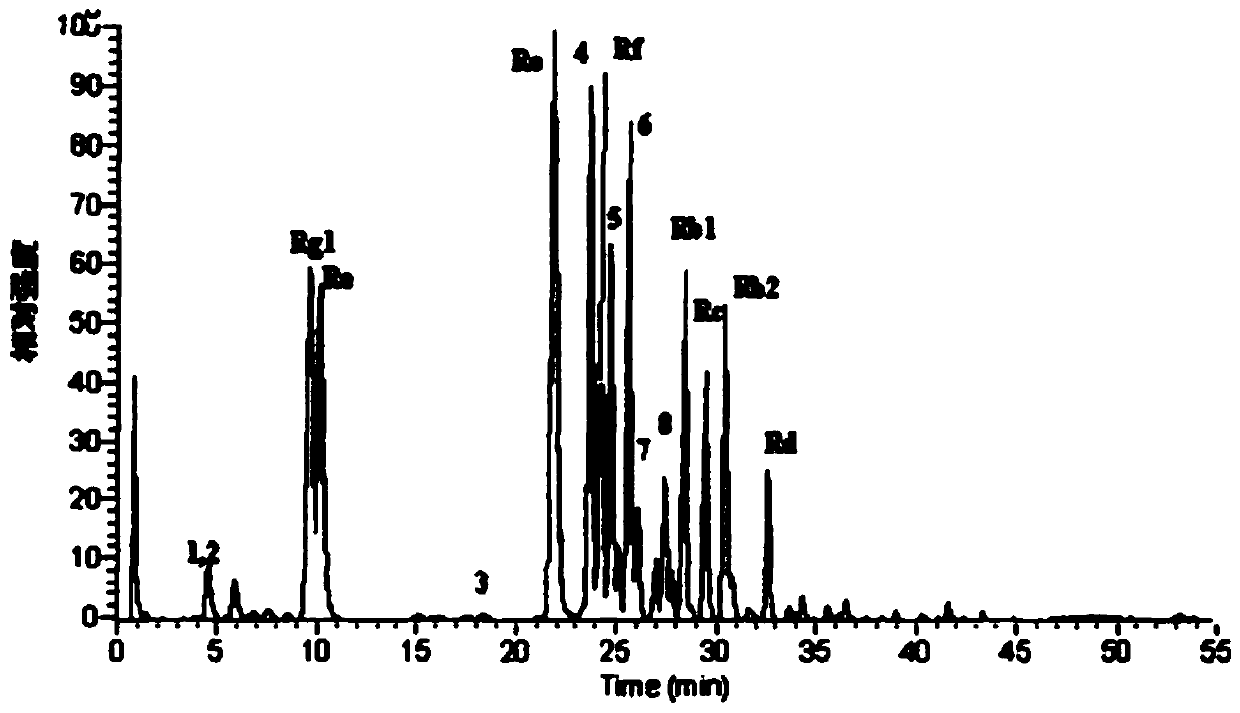
[0135] A freeze-dried preparation, which is used as an oral preparation, the triterpene saponin in the freeze-dried preparation is malonyl ginsenoside Re, and its percentage content is 10%, the binder is pullulanase polysaccharide, and its The percentage content is 3%.
[0136] The freeze-dried preparation is prepared through the following steps:
[0137] a) After mixing water, malonyl ginsenoside Re and pullulanase polysaccharide to form a pre-preparation solution, constant volume and degassing;
[0138] b) Using a quantitative filling pump, inject the pre-dosing solution obtained in a) into a 1ml sheet-shaped molding mold for degassing;
[0139] c) freeze-drying the side product obtained in b), and removing the solvent to obtain an oral freeze-dried preparation.
[0140] The oral preparation has the function of lowering blood sugar, the disintegration time is within 3s, and the dissolution rate is 95% within 30s. In this example, malonyl ginsenoside Re was replaced with oth...
Embodiment 2
[0143] A freeze-dried preparation, which is used as an oral preparation, the triterpenoid saponins in the freeze-dried preparation are 10% malonyl ginsenoside Rg1 and 20% malonyl ginsenoside Rb1, the total is 30%, and the binder It is guar gum (0.5%) and butylene glycol (15%); the auxiliary material is a skeleton support agent (modified tapioca starch), and its percentage is 5%. The disintegration time of the oral preparation is within 3s, and the dissolution rate is 95% within 30s.
[0144] The freeze-dried preparation is prepared through the following steps:
[0145] a) Preparation of soft ice mixture: mix malonyl ginsenoside Rg1 and malonyl ginsenoside Rb1, guar gum and butylene glycol with water according to a set ratio to obtain a pre-preparation solution, and freeze the pre-preparation solution to obtain soft ice mix 1;
[0146] b) Use a spherical mold with a specification of 0.6ml to shape the shape, obtain the shaped mixture 2, and demould;
[0147] c) Freeze-drying...
Embodiment 3
[0151] A kind of freeze-dried preparation, this freeze-dried preparation is used as oral preparation, and triterpene saponin is platycodon saponin E30% in this freeze-dried preparation, and binding agent is modified starch (percentage content is 30%); Adjuvant is antioxidant ( Vitamin C, its percentage content is 7%), and active ingredient is ginseng polysaccharide (percentage content is 5%). The disintegration time of the oral preparation is within 3s, and the dissolution rate is 90% within 50s.
[0152] The freeze-dried preparation is prepared through the following steps:
[0153] a) Preparation of soft ice mixture: mixing platycodon saponin E, modified starch, vitamin C and water according to a set ratio to obtain a pre-preparation liquid, freezing the pre-preparation liquid to obtain a soft ice mixture;
[0154] b) using ginseng polysaccharide as dry powder;
[0155] e) mixing soft ice mixture and ice powder to obtain all soft ice mixtures;
[0156] f) use an ellipsoida...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com