Fluorescent labeled organic boron/nitrogen Lewis acid and base bifunctional catalyst and preparation method thereof
A dual-functional catalyst, acid-base dual-functional technology, applied in organic chemical methods, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of no catalytic activity, expensive raw materials, single method, etc. problem, to achieve good non-enantiomeric selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
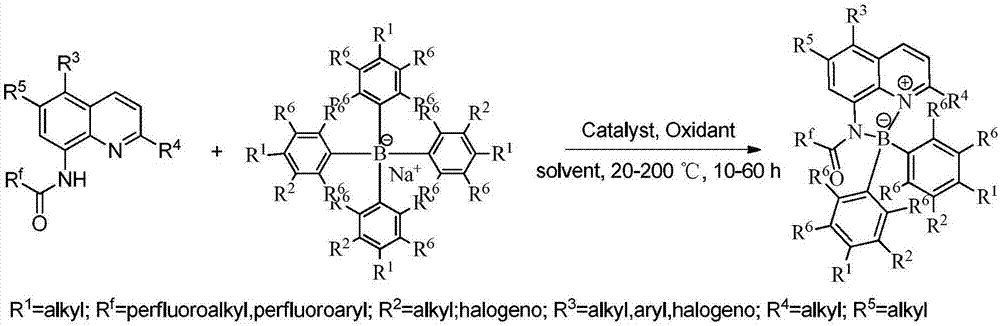
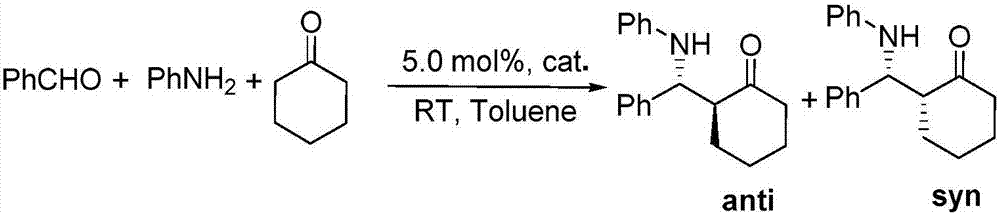
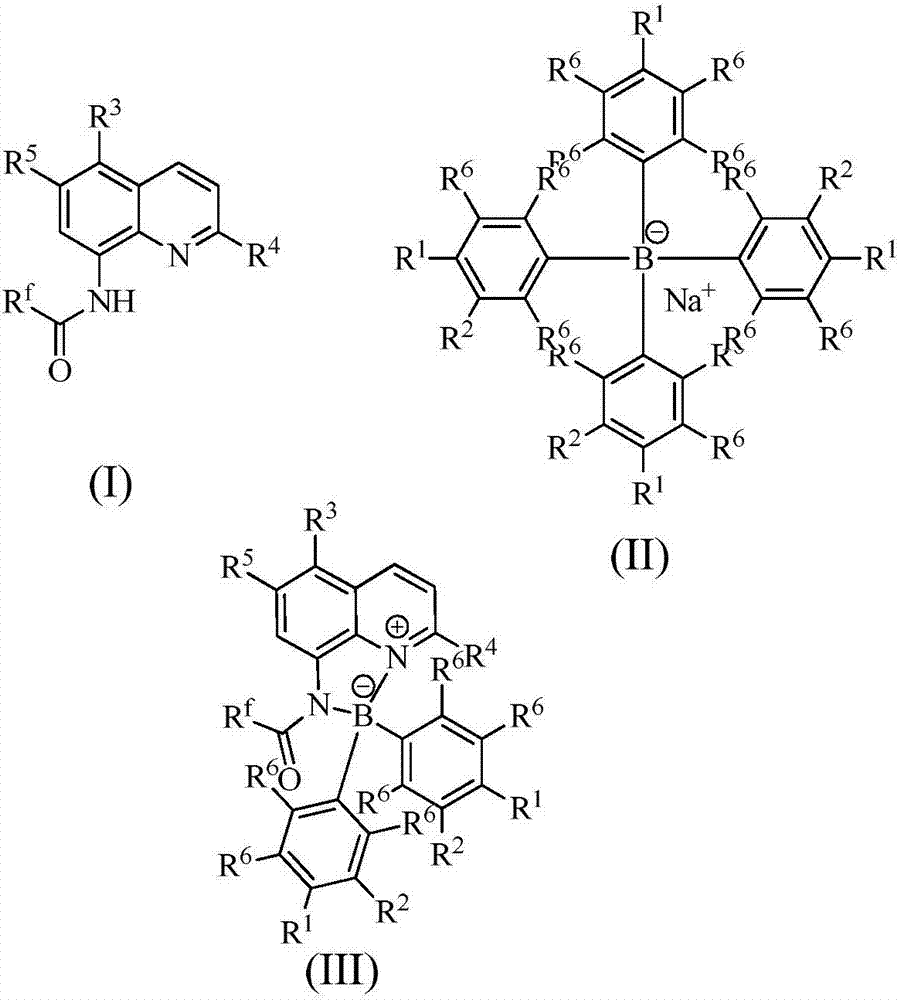
[0027] Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.2mmol II (wherein R 1 , R 2 , R 6 = H), 0.005 mmol I 2 and 1mL toluene, under a nitrogen atmosphere, the reaction was carried out at 150°C for 12h. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography to obtain III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), the productive rate is 96%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol cyclohexanone, 0.002mmol III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H) and 1mL toluene, the reaction was carried out at 25°C for 12h, and TLC followed the reaction until the reaction was complete. The reaction result was: 2-anilinobenzylcyclohexanone, the productive rate was 78%, and the cis-trans selectivity was 1 / 99.
preparation example 2
[0029] Add 0.1 mmol I (wherein R f =C 2 f 5 ; 3 , R 4 , R 5 =H), 0.2mmol II (wherein R 1 = H, R 2 , R 6 = H), 0.005 mmol I 2 and 1mL toluene, under a nitrogen atmosphere, the reaction was carried out at 150°C for 12h. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography to obtain III (wherein R f =C 2 f 5 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6=H) yield is 73%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol cyclohexanone, 0.002mmol III (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H) and 1mL toluene, the reaction was carried out at 25°C for 12h, and TLC followed the reaction until the reaction was complete. The reaction result was: 2-anilinobenzylcyclohexanone, the productive rate was 83%, and the cis-trans selectivity was 1 / 99.
preparation example 3
[0031] Add 0.1 mmol I (wherein R f = CF 3 ; 3 , R 4 , R 5 =H), 0.2mmol II (wherein R 1 , R 6 = H; R 2 =OMe), 0.005mmol I 2 and 1mL toluene, under a nitrogen atmosphere, the reaction was carried out at 150°C for 12h. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography to obtain III (wherein R f = CF 3 ; 2 =OMe;R 1 , R 3 , R 4 , R 5 , R 6 =H), the productive rate is 60%. Detection of catalytic activity: add 0.5mmol aniline (Ar=Ph), 0.5mmol benzaldehyde (Ar=Ph), 0.6mmol cyclohexanone, 0.002mmol III (wherein R f = CF 3 ; 2 =OMe;R 1 , R 3 , R 4 , R 5 , R 6 =H) and 1mLtoluene, the reaction was carried out at 25°C for 12h, TLC followed the reaction until the reaction was complete. The reaction result was: 2-anilinobenzylcyclohexanone, the yield was 83%, and the cis-trans selectivity was 1 / 99 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com