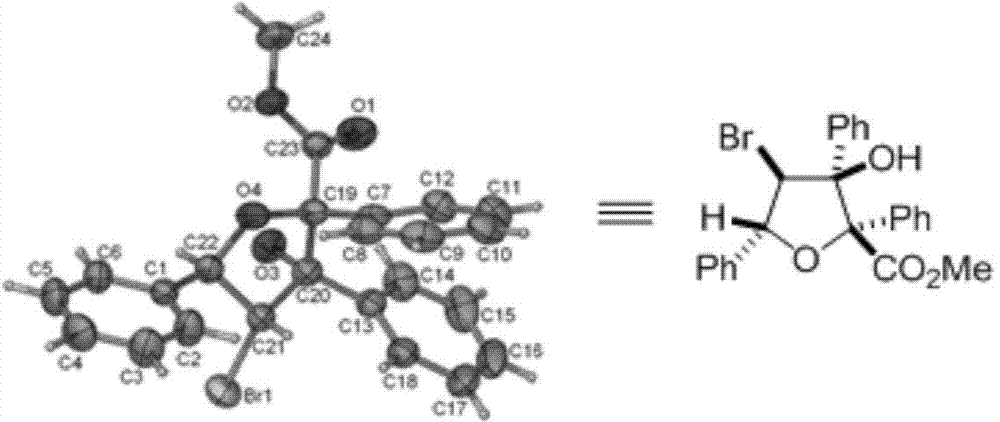
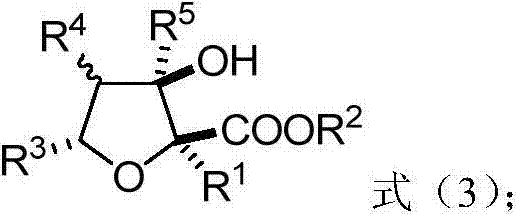
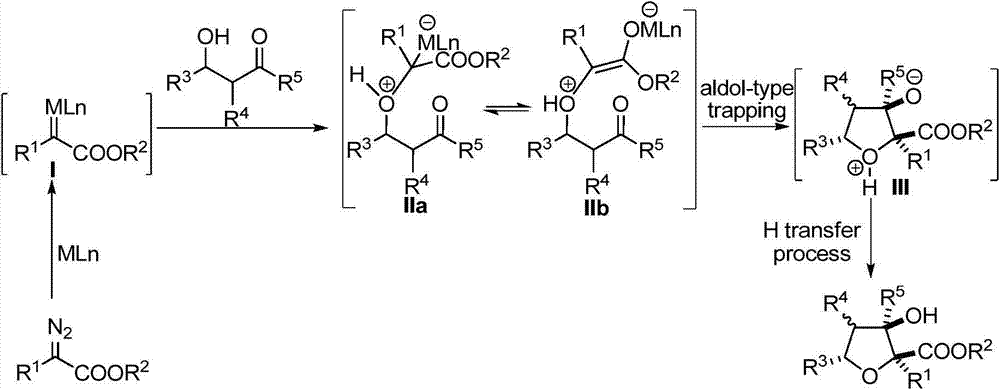
Polysubstituted tetrahydrofuran derivatives as well as synthesis method and application thereof
A technology of tetrahydrofuran and a synthetic method is applied in the field of preparation and application of pharmaceutical intermediates, and can solve the problems of many reaction steps, complicated operation and post-processing, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]
[0062] The 3-hydroxy-1,3-diphenylpropan-1-one (0.30mmol, 1.0eq.), Molecular sieve (300mg) and rhodium acetate (0.003mmol) were dissolved in dichloromethane (1.5ml) at 25℃; then, phenyldiazomethyl ester (0.33mmol) dissolved in dichloromethane (1.5ml) , 1.1eq.) was added dropwise to the reaction system within 1h at 25°C. After the addition was completed, the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate: petroleum ether = 1:30 to 1:10) to obtain a pure product. The yield is 80%, and the dr value is greater than 95:5.
[0063] 1 H NMR(400MHz, CDCl 3 )δ7.63(d, J=7.5Hz, 2H), 7.45(t, J=7.5Hz, 2H), 7.36(t, J=7.3Hz, 1H), 7.19(d, J=7.4Hz, 2H) , 7.10-7.00 (m, 8H), 5.74 (dd, J = 10.6, 5.2 Hz, 1H), 4.53 (s, 1H), 3.79 (s, 3H), 2.74-2.69 (m, 1H), 2.59 (dd , J=12.8, 5.2Hz, 1H); 13 C NMR(100MHz, CDCl 3 )δ173.62,140.77,140.18,137.96,128.61,127.88,127.63,127.39,127.31,1...
Embodiment 2
[0065]
[0066] The 3-hydroxy-1,3-diphenylpropan-1-one (0.30mmol, 1.0eq.), Molecular sieve (300mg) and palladium acetate (0.006mmol) were dissolved in toluene (1.5ml) at 0°C; then, phenyldiazomethyl ester (0.36mmol, 1.2eq.) dissolved in toluene (1.5ml). ) Add dropwise to the reaction system at 0°C within 1 hour, after the dropwise addition is completed, the solvent is removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate: petroleum ether = 1:30 to 1:10) to obtain a pure product. The yield is 84%, and the dr value is greater than 95:5.
[0067] 1 H NMR(400MHz, CDCl 3 )δ7.63(d, J=7.5Hz, 2H), 7.45(t, J=7.5Hz, 2H), 7.36(t, J=7.3Hz, 1H), 7.19(d, J=7.4Hz, 2H) , 7.10-7.00 (m, 8H), 5.74 (dd, J = 10.6, 5.2 Hz, 1H), 4.53 (s, 1H), 3.79 (s, 3H), 2.74-2.69 (m, 1H), 2.59 (dd , J=12.8, 5.2Hz, 1H); 13 C NMR(100MHz, CDCl 3 )δ173.62,140.77,140.18,137.96,128.61,127.88,127.63,127.39,127.31,127.21,126...
Embodiment 3
[0069]
[0070] The 3-hydroxy-1,3-diphenylpropan-1-one (0.30mmol, 1.0eq.), Molecular sieve (300mg) and rhodium acetate (0.006mmol) were dissolved in chloroform (1.5ml) at 40℃; then, phenyldiazomethyl (0.42mmol) dissolved in chloroform (1.5ml) , 1.4eq.) was added dropwise to the reaction system within 1h at 40°C, and after the addition was completed, the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate: petroleum ether = 1:30 to 1:10) to obtain a pure product. The yield was 87%, and the dr value was greater than 95:5.
[0071] 1 H NMR(400MHz, CDCl 3 )δ7.63(d, J=7.5Hz, 2H), 7.45(t, J=7.5Hz, 2H), 7.36(t, J=7.3Hz, 1H), 7.19(d, J=7.4Hz, 2H) , 7.10-7.00 (m, 8H), 5.74 (dd, J = 10.6, 5.2 Hz, 1H), 4.53 (s, 1H), 3.79 (s, 3H), 2.74-2.69 (m, 1H), 2.59 (dd , J=12.8, 5.2Hz, 1H); 13 C NMR(100MHz, CDCl 3 )δ173.62,140.77,140.18,137.96,128.61,127.88,127.63,127.39,127.31,127.21,126....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com