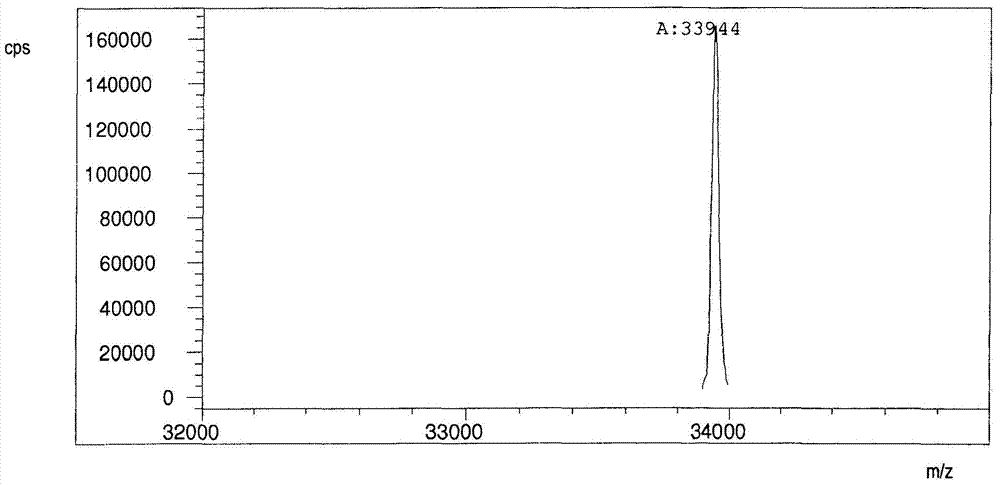
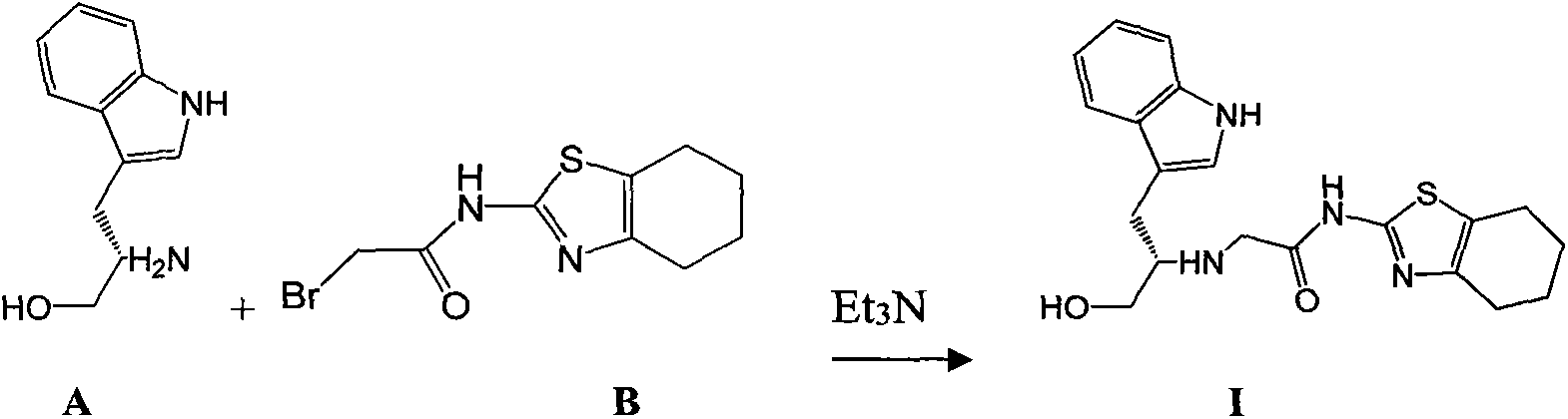
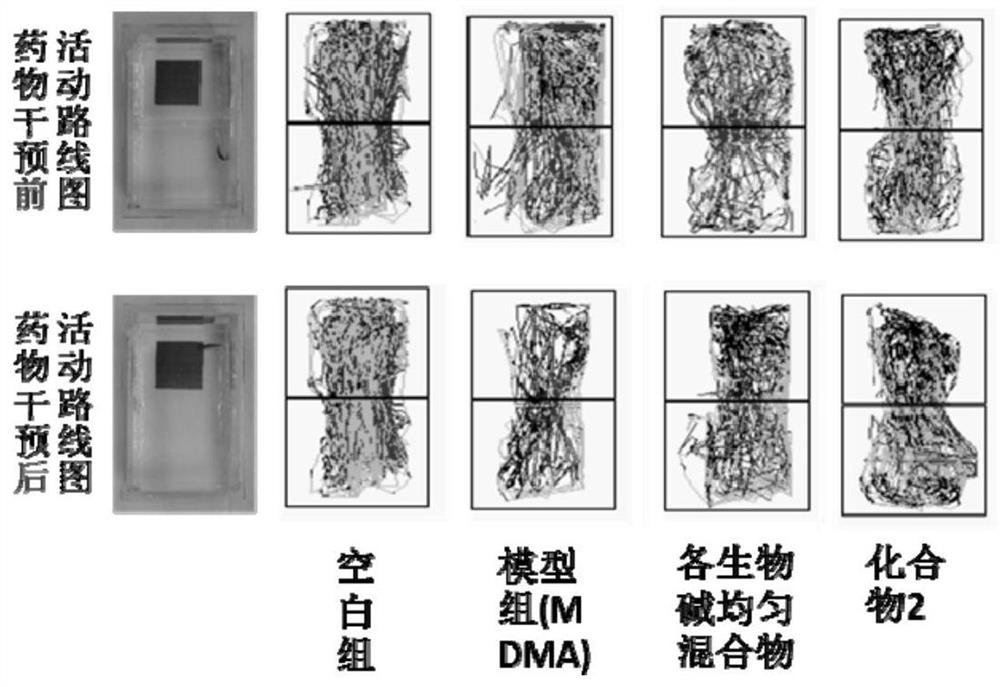
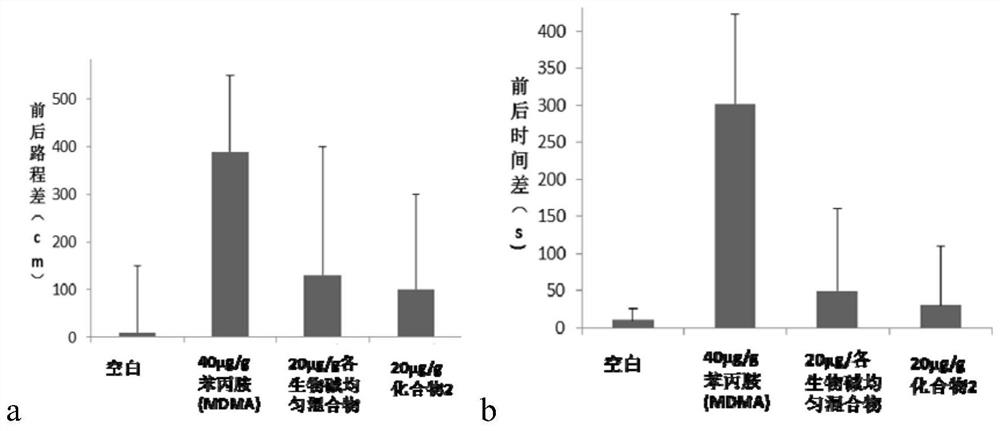
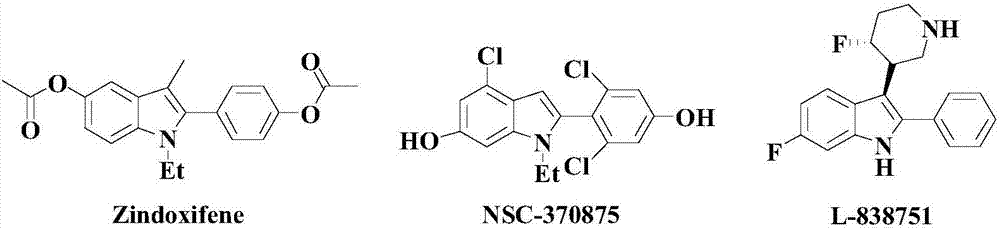
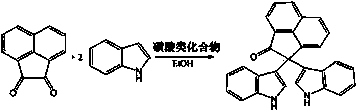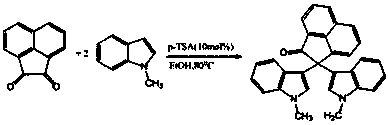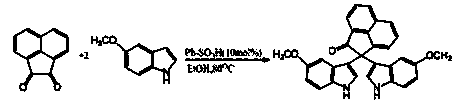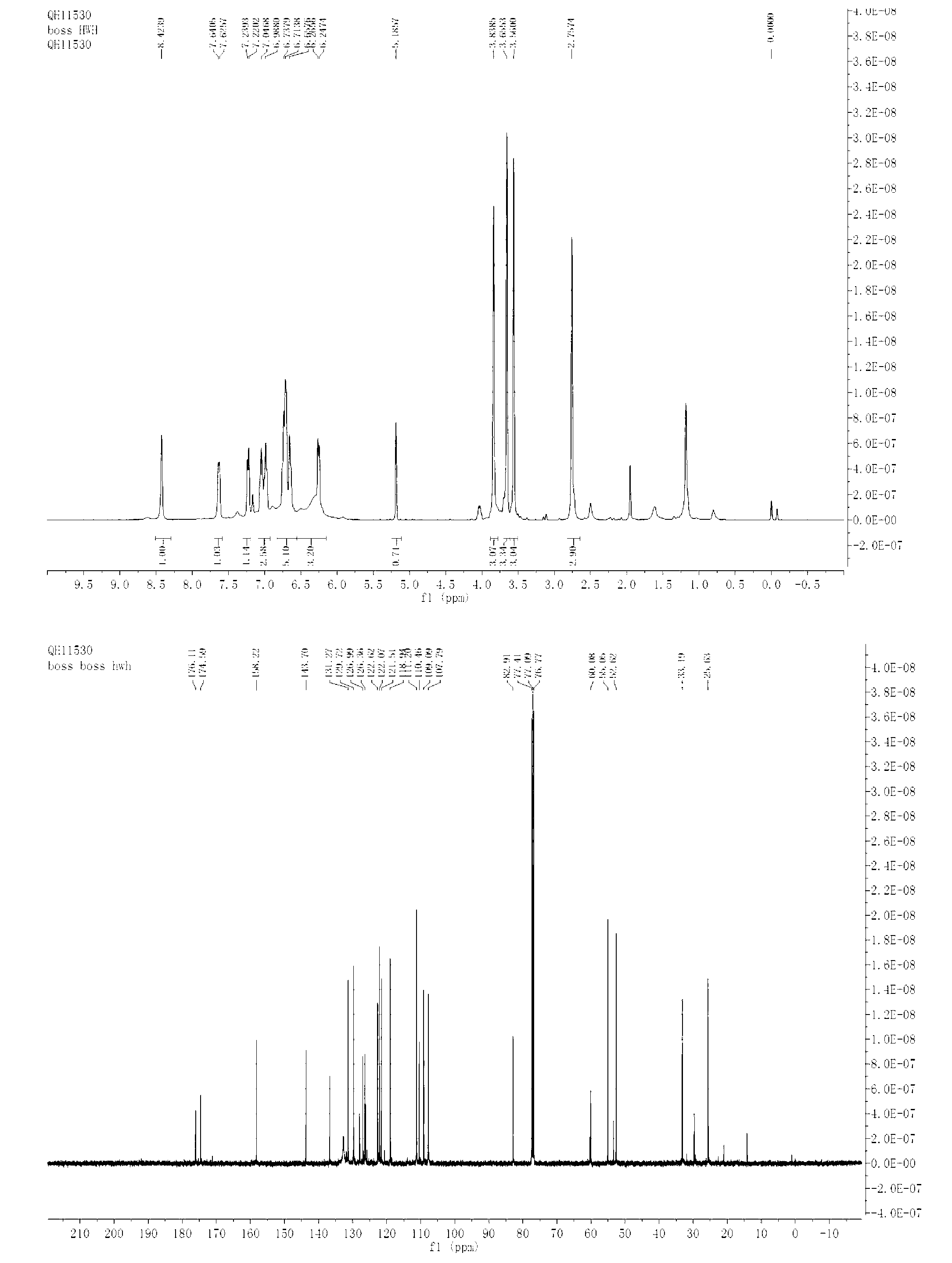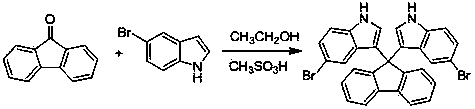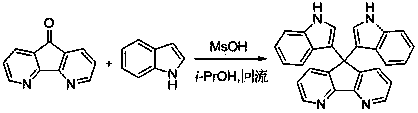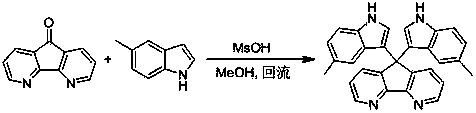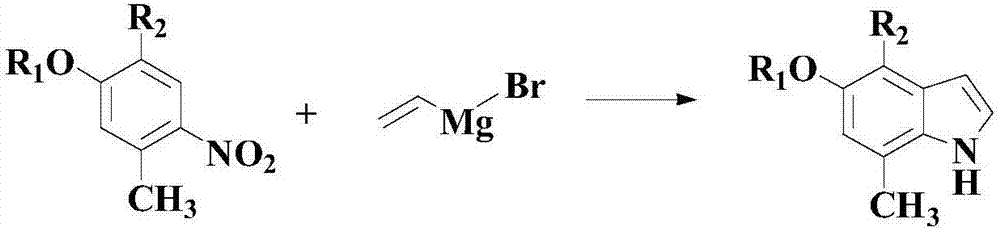Patents
Literature
88 results about "Bis indole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment of protein folding disorders
InactiveUS20070015813A1Increased Design PossibilitiesBiocideNervous disorderMedicineCompound (substance)
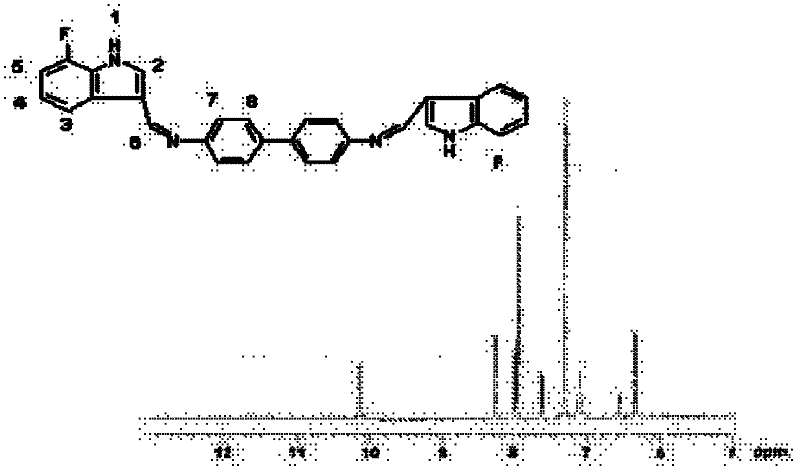
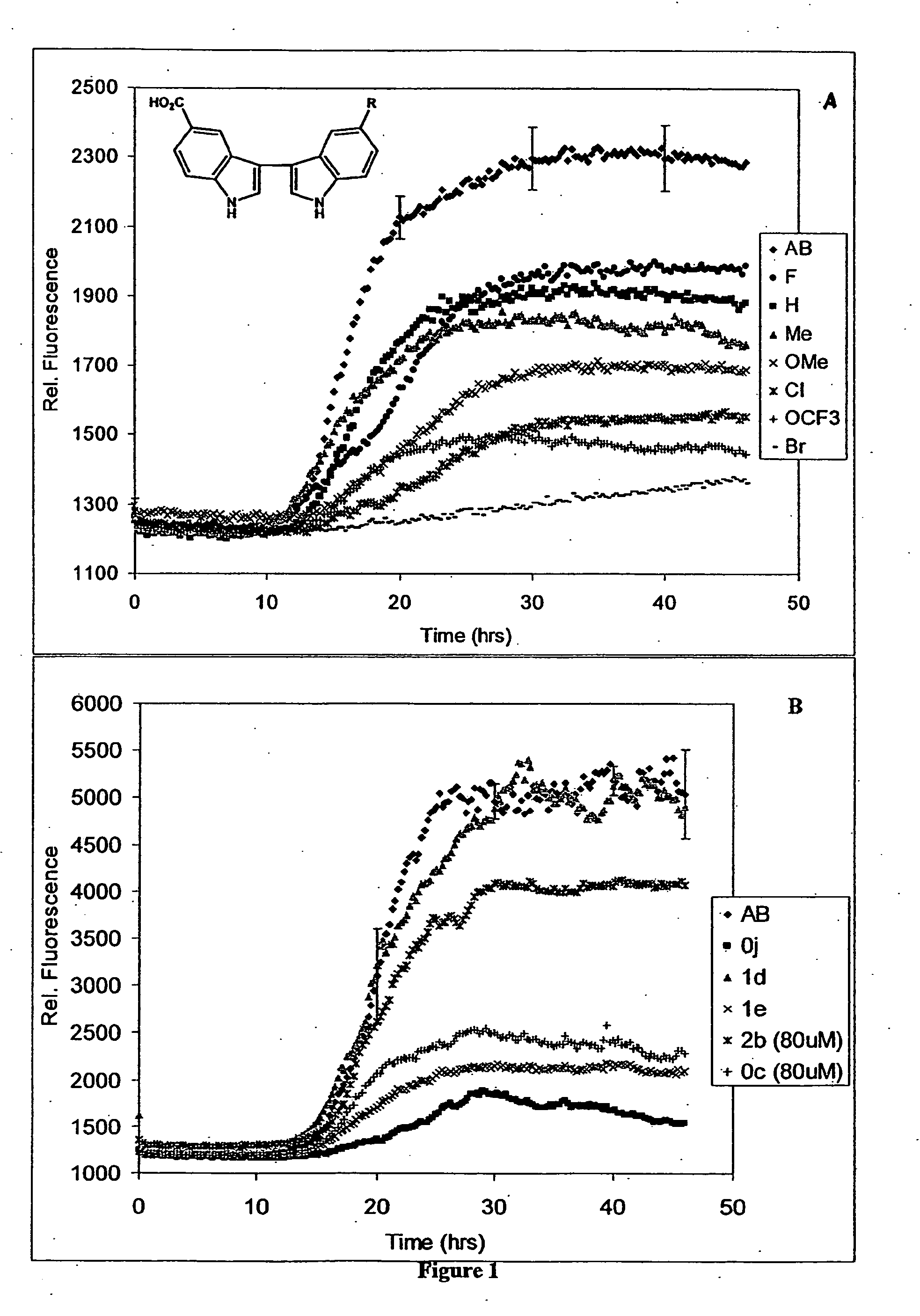
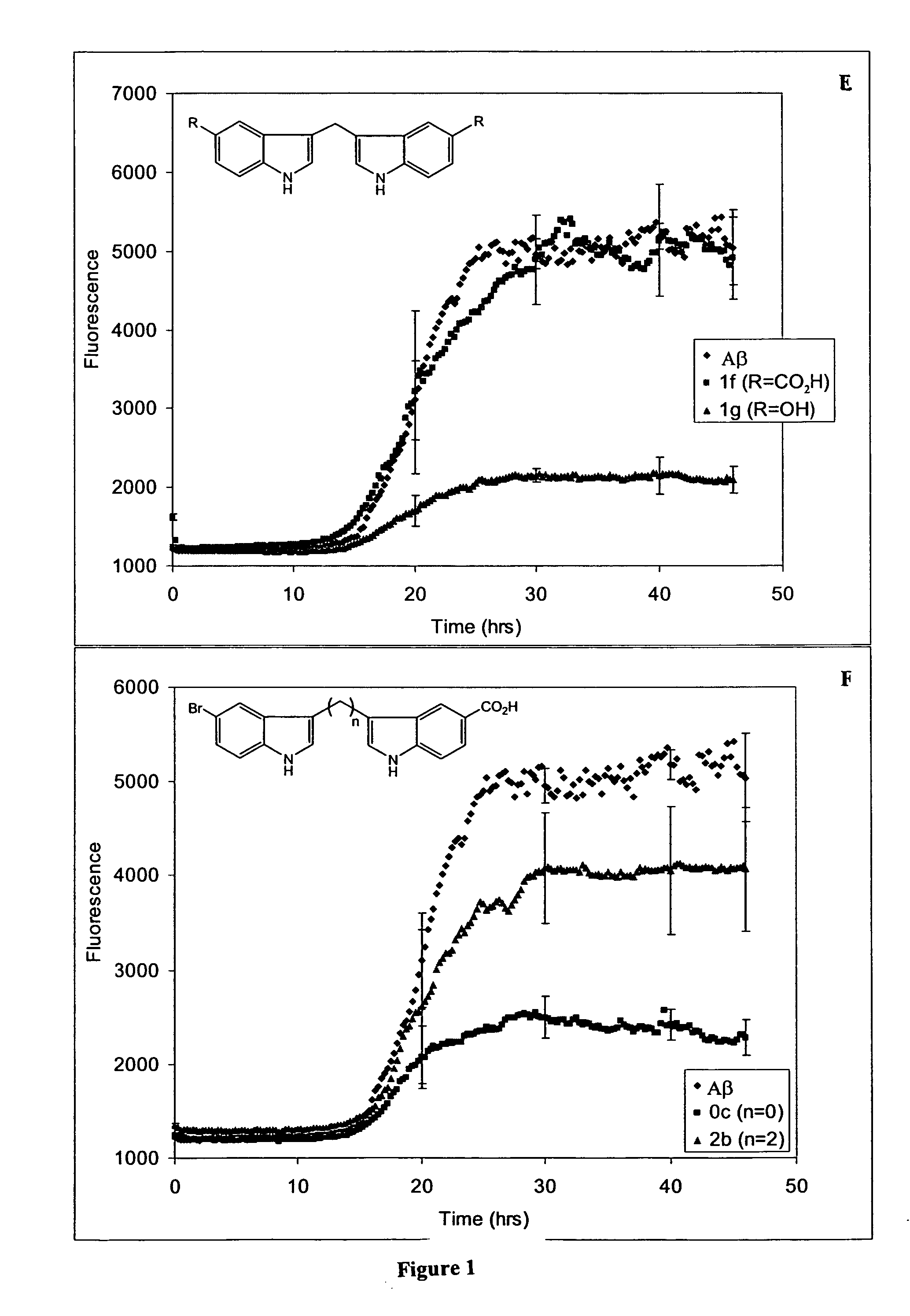
In certain embodiments, the invention is directed to a method for treating a protein folding disorder comprising administering to a subject a compound of the formulas disclosed. In preferred embodiments, the compounds are bis-indole compounds.
Owner:TREVENTIS CORP
Organic electroluminescent device
InactiveUS20110062862A1Improve luminous efficiencySecuring drive stabilityOrganic chemistryDischarge tube luminescnet screensDopantHost material
Disclosed is an organic electroluminescent device (organic EL device) that is improved in the luminous efficiency, fully secured of the driving stability, and of a simple structure. The organic EL device comprises a light-emitting layer between an anode and a cathode piled one upon another on a substrate and the said light-emitting layer comprises (A) a phosphorescent dopant whose emission peak wavelength is longer than 600 nm and (B) a host material. The host material contains at least two kinds of compounds selected from two or more kinds of derivatives included in (b1) N-substituted indolocarbazole derivatives, (b2) derivatives of 8-hydroxyquinoline aluminum complex, and (b3) bisindolocarbazole derivatives.
Owner:PIONEER CORP +2
Compound of biindole heterocycles, preparation method and application in use for organic electroluminescence material
InactiveCN1562999ASimple preparation processHigh yieldOrganic chemistryLuminescent compositionsOrganic solventHole transport layer
This invention relates to metal for prepn. of bis-indole heterocycle type organic electroluminescence material with characteristic of having indok-aromatic heterocycle-indole structure. advantages are: easy synthesis and purification, high opto-thermo stability, easy to be dissolved in organic solvent, high fluorescent light, high-hole conduction property, being used as hole conduction layer and luminous layer of OLED. In the prepn., indole boric acid or its ester and dihalogenated aromatic hydrocarbon or dihalogenated aromatic heterocycle compound are proceed Suzuki coupling reaction to obtain said product.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
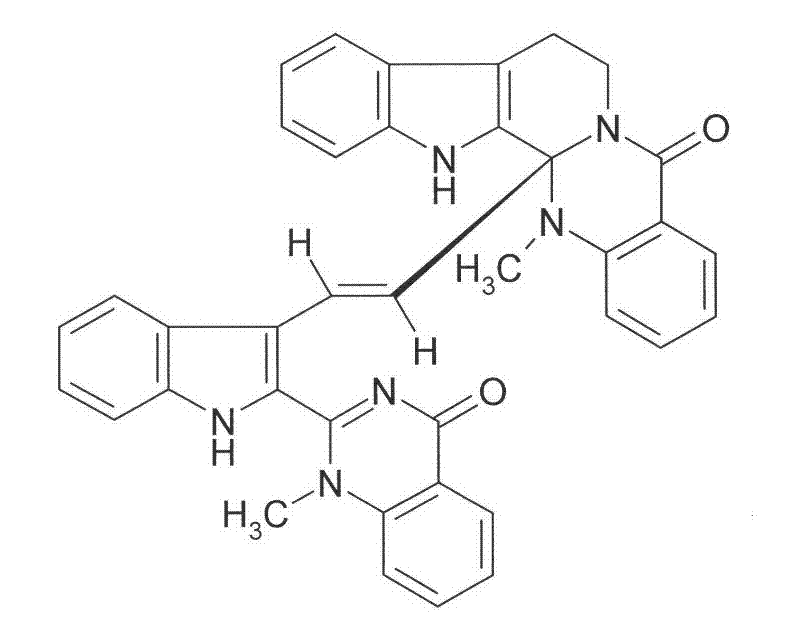
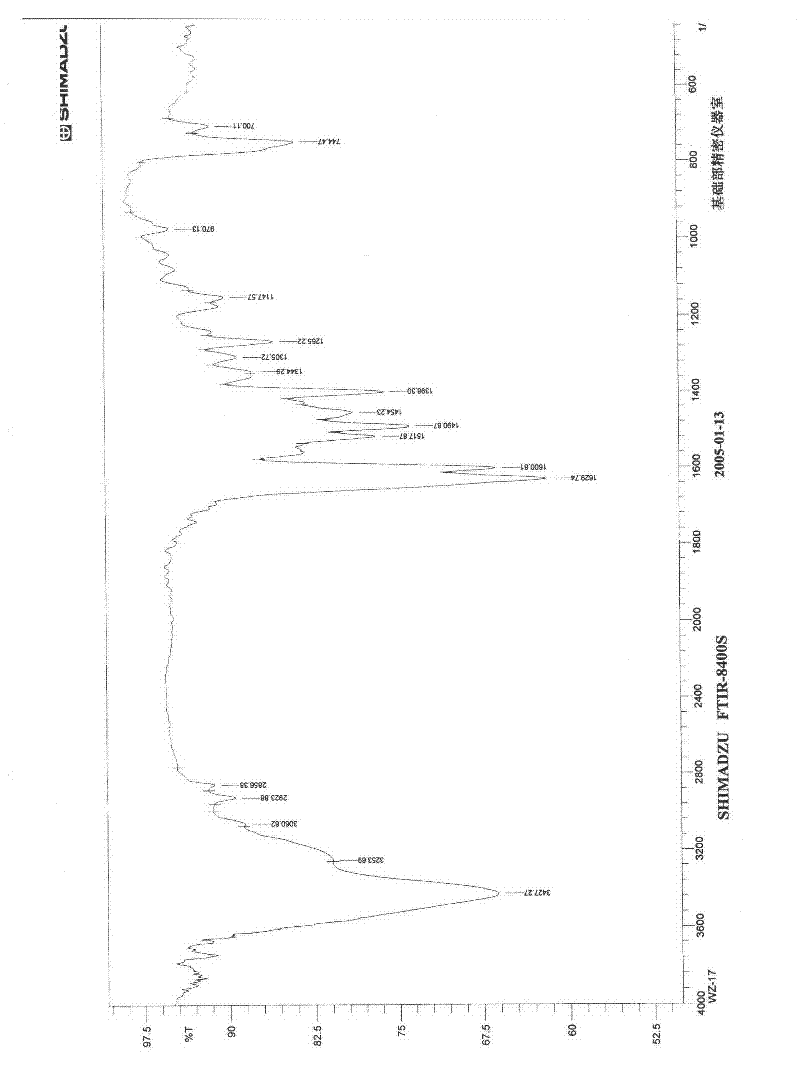
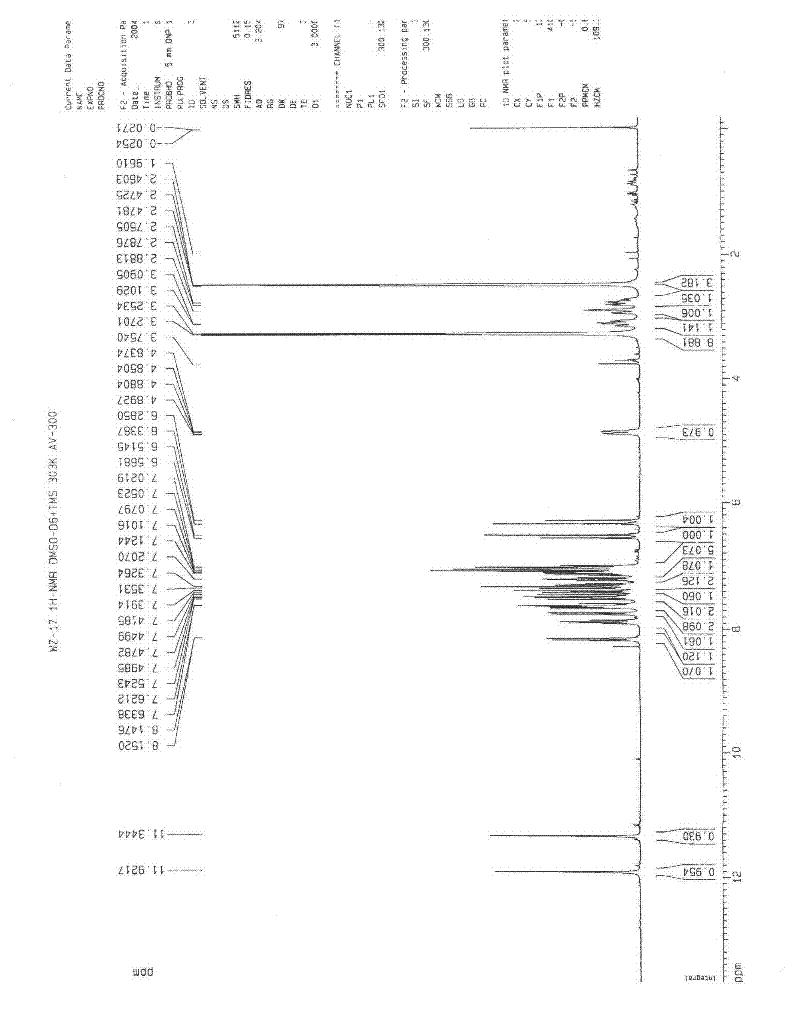
Application of diindoloquinazoline alkaloid in preparation of antitumor drugs and antifungal drugs
The present invention relates to a diindoloquinazoline alkaloid and diindoloquinazoline alkaloid derivatives. The structure of the diindoloquinazoline alkaloid is represented by the right formula. The diindoloquinazoline alkaloid is a dimmer compound formed by linkage of two different indoloquinazoline alkaloids with C-C bonds. The present invention further relates to a preparation method for the compounds, and antitumor activities and antifungal activities of the compounds. The diindoloquinazoline alkaloid and the diindoloquinazoline alkaloid derivatives of the present invention can be applicable for the field of medicine.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Bis-indole pyrroles useful as antimicrobials agents
Compounds of Formula I, commonly referred to as bis-indole pyrroles, including isolated naturally-occurring compounds, synthetic and semi-synthetic derivatives thereof having antimicrobial properties and to antimicrobial compositions that include one or more of bis-indole pyrroles and their derivatives or analogs having antimicrobial properties are disclosed. Pharmaceutical compositions comprising such compounds and methods of treating bacterial infections with the disclosed compounds or the disclosed pharmaceutical compositions are also disclosed.
Owner:NEREUS PHARMA
Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole
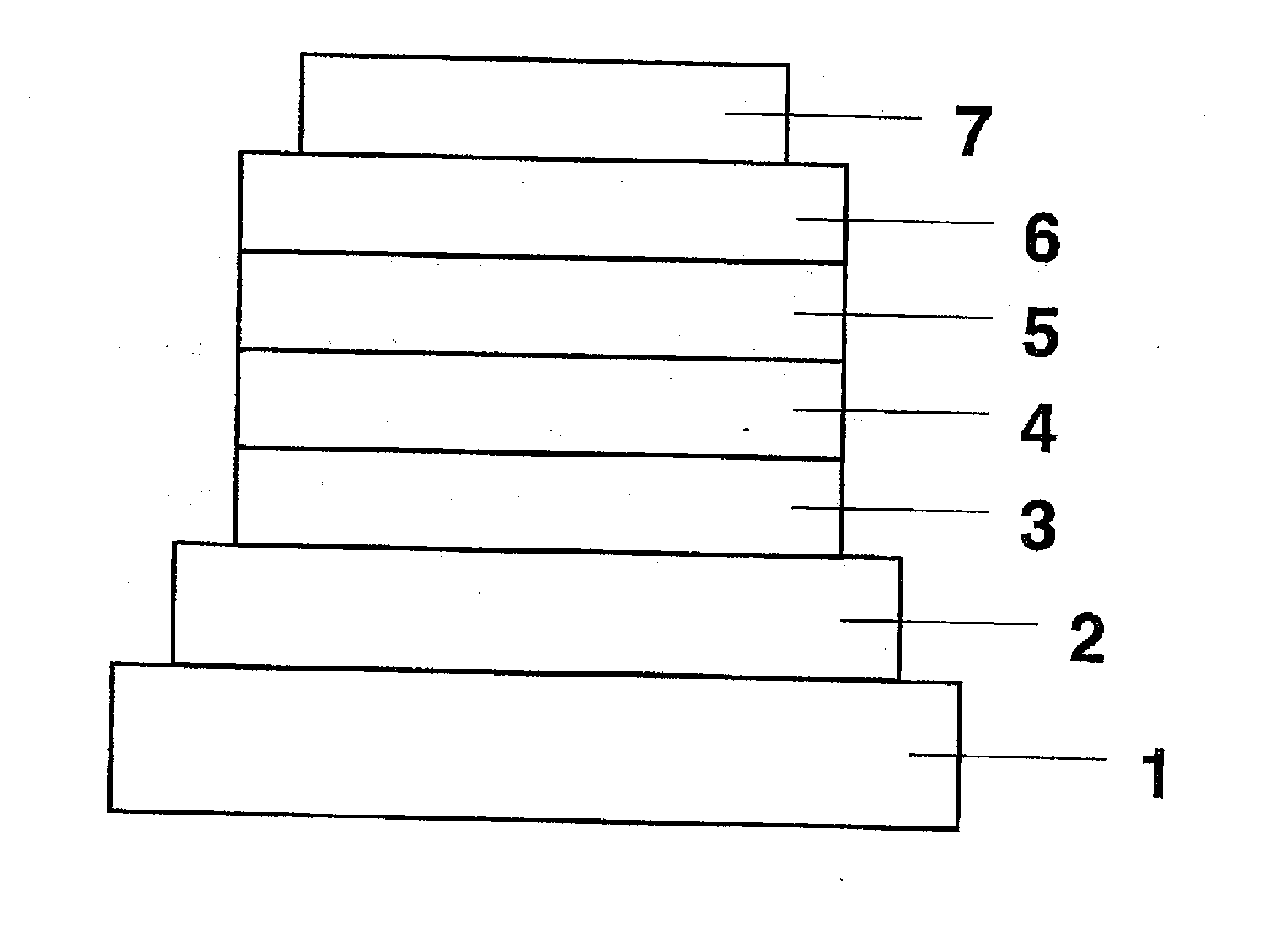
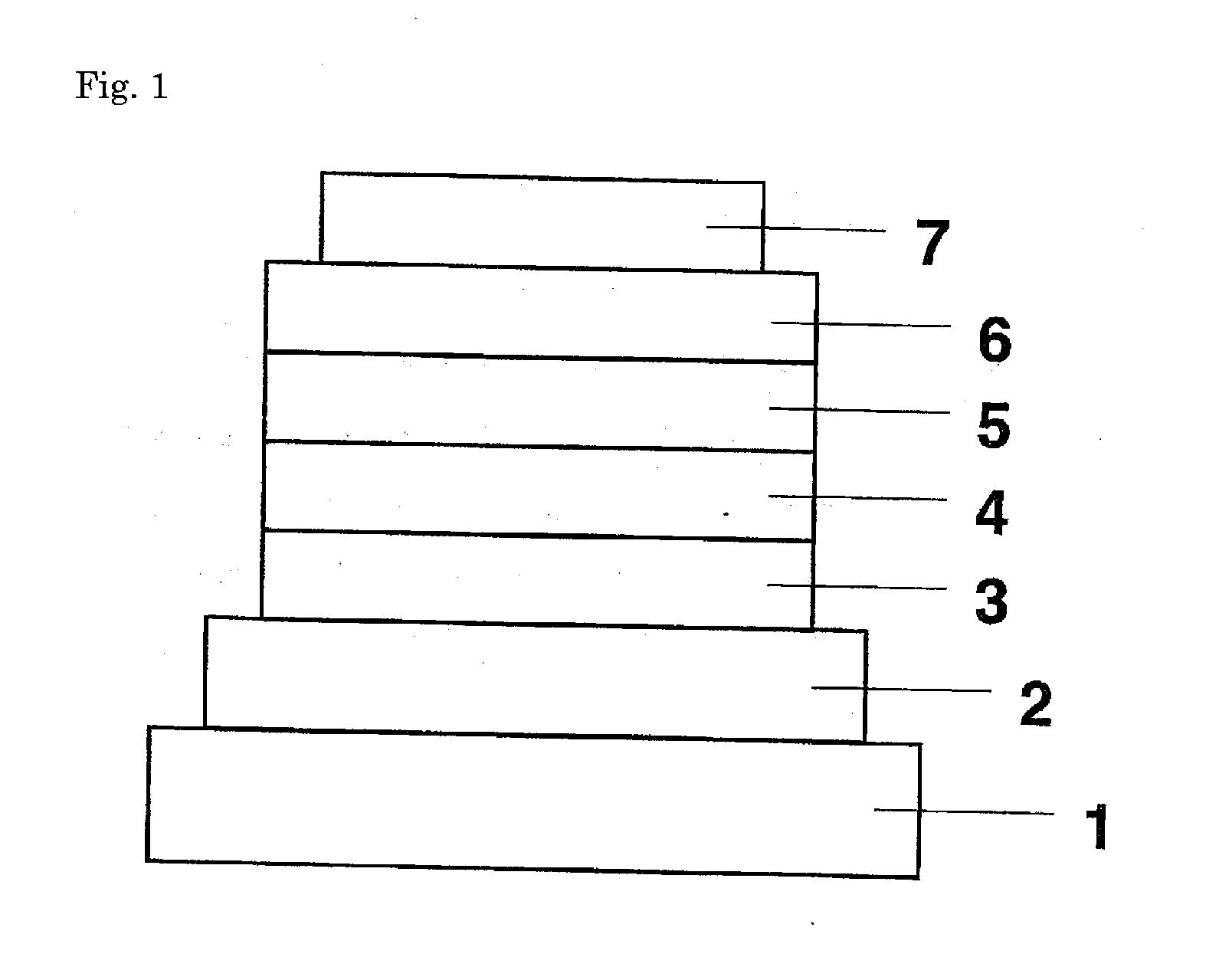
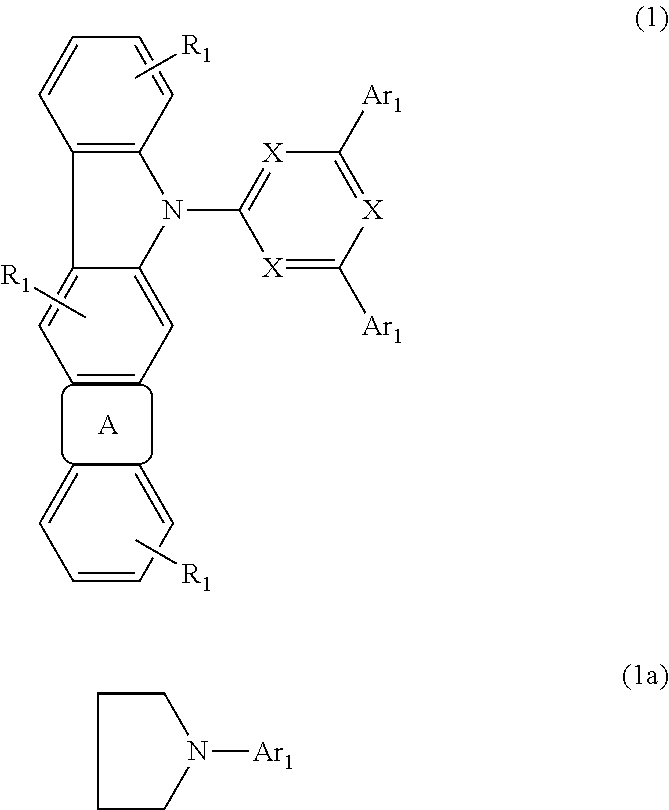
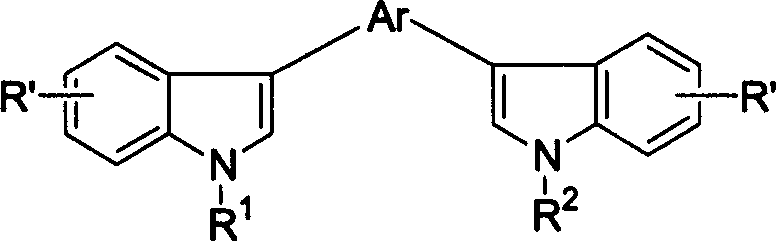
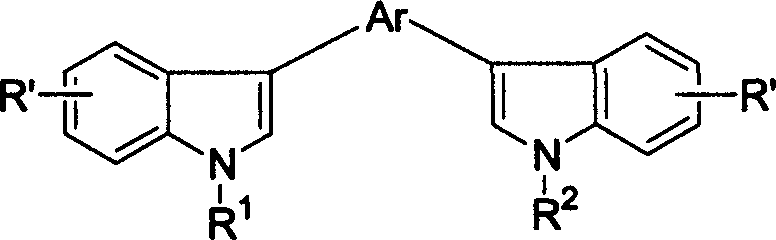
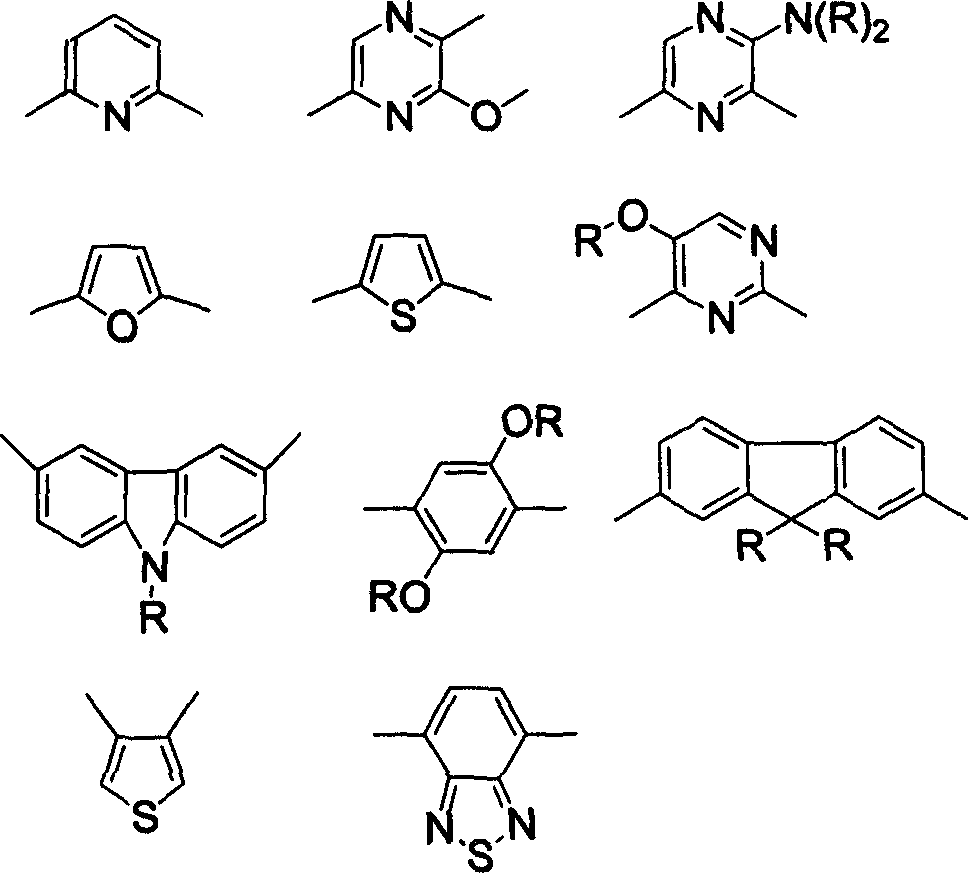
ActiveUS8426850B2Optimization rangeImprove thermal stabilityGroup 8/9/10/18 element organic compoundsSolid-state devicesArylDopant
The present invention relates to an organic semiconductor element that comprises multiple layers. One or more layers may include compounds that can function as light absorbers, charge transporting materials, and / or as a dopant.
Owner:BASF AG +1
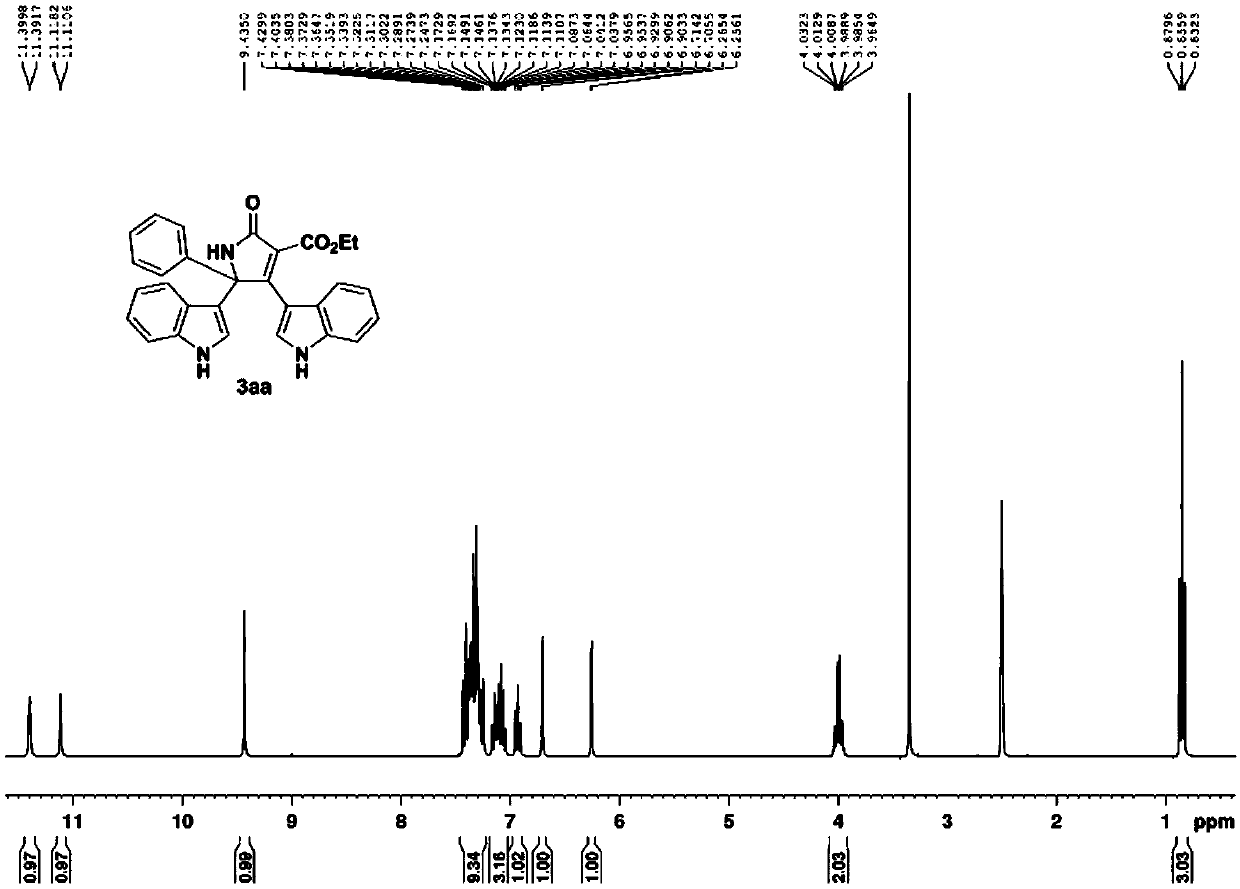
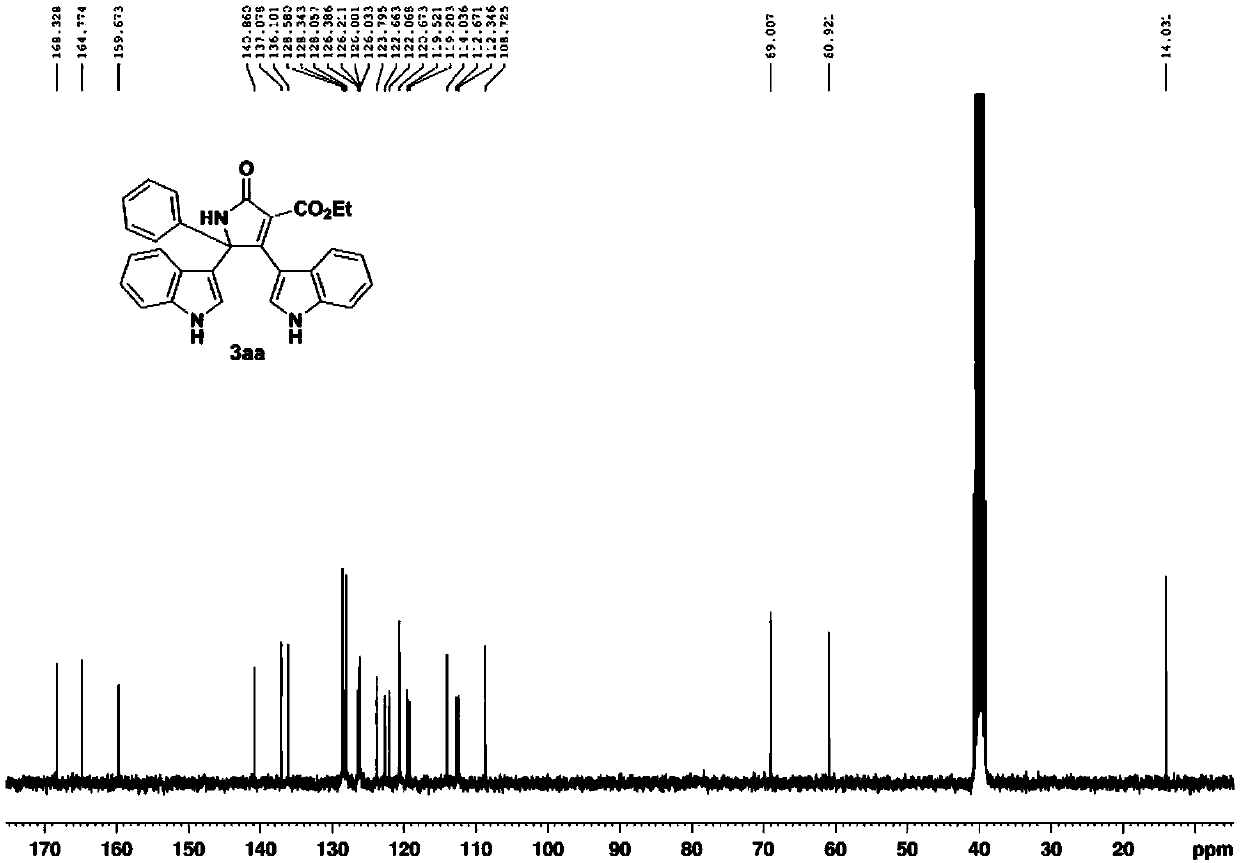
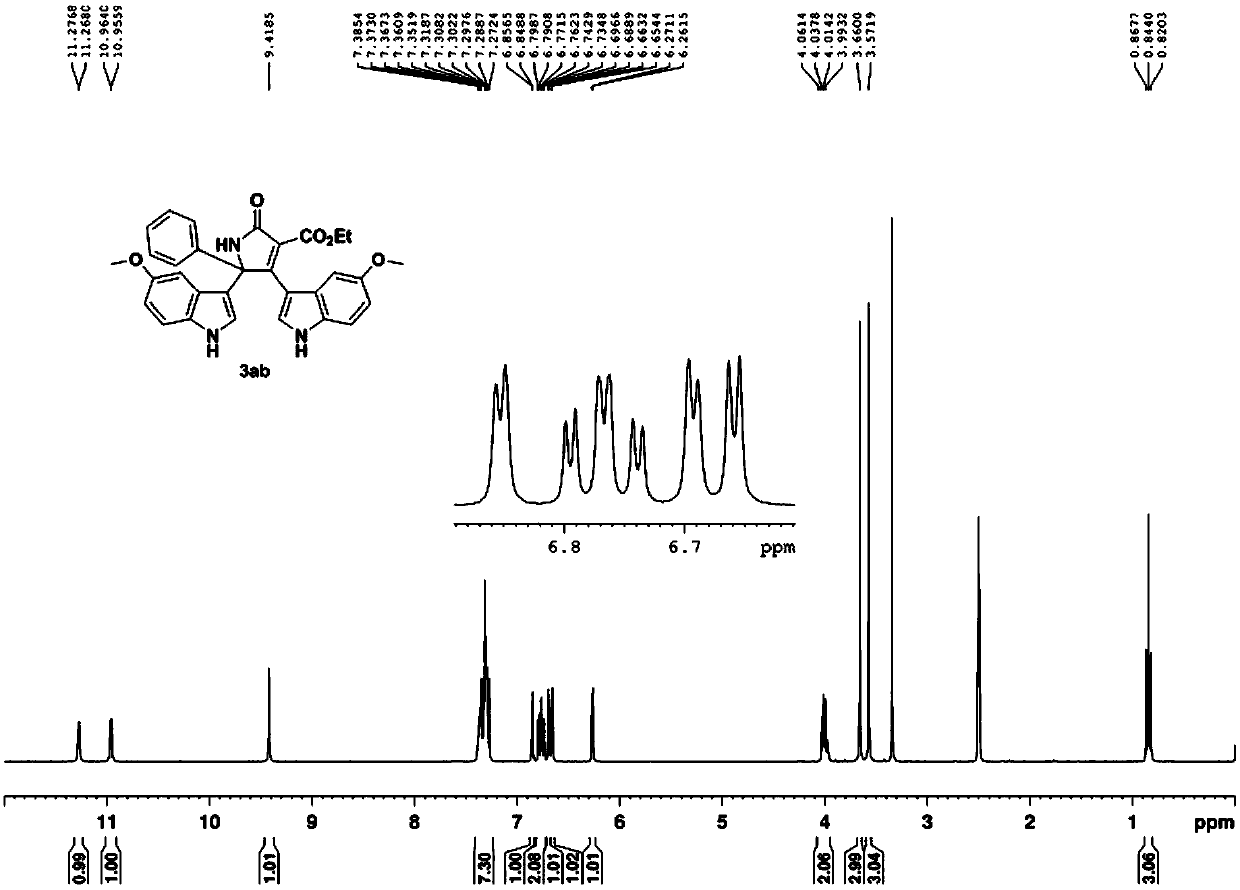
Method for constructing bisindole substituted dihydropyrrole derivative based on oxime ester and indole
The invention belongs to the field of organic chemical industry, and particularly relates to a method for constructing a bisindole substituted dihydropyrrole derivative based on oxime ester and indole. The method comprises the steps that diethyl malonate substituted ketoxime acid ester, an indole compound and an oxidizing agent serve as raw materials and are heated to 60-100 DEG C under the protection condition of N2, and reaction is carried out so as to obtain the bisindole substituted dihydropyrrolidone derivative. According to the method, the indole compound, the diethyl malonate substituted ketoxime acid ester and the oxidizing agent are selected, the raw materials are cheap and easy to obtain, substrates are wide in range, the compatibility of functional groups is good, and the finalpyrrolidone derivative can be obtained with relatively high yield through various substituted indole and the diethyl malonate substituted ketoxime acid ester. The method has the advantages that the synthesis route is short, the toxicity of the reaction raw materials is low, separation and purification are convenient, reaction liquid can be obtained through recrystallization only, and the method issuitable for large-scale industrial production and has an important practical application value in a method for synthesis of the dihydropyrone derivative.
Owner:CHANGZHOU UNIV
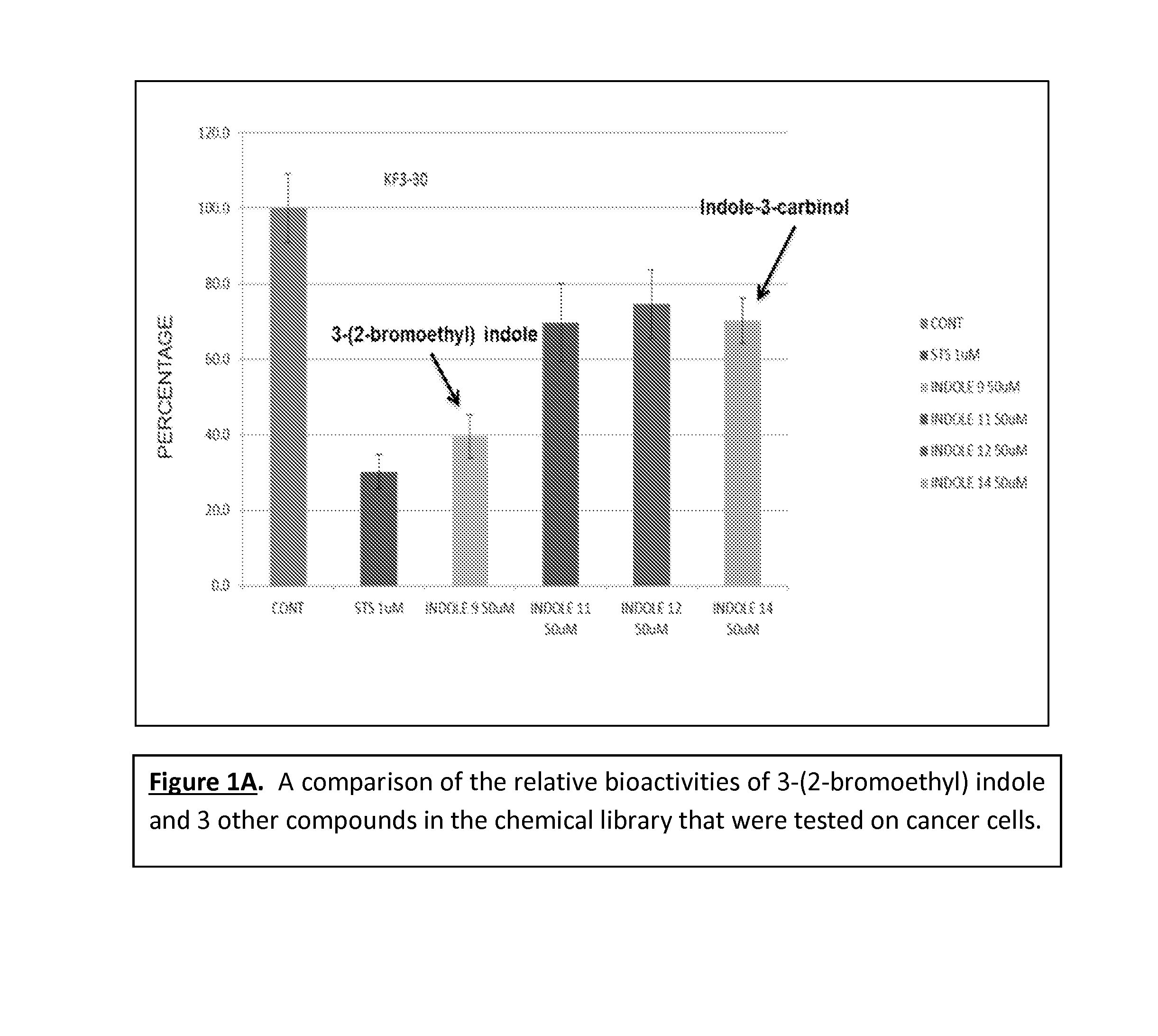
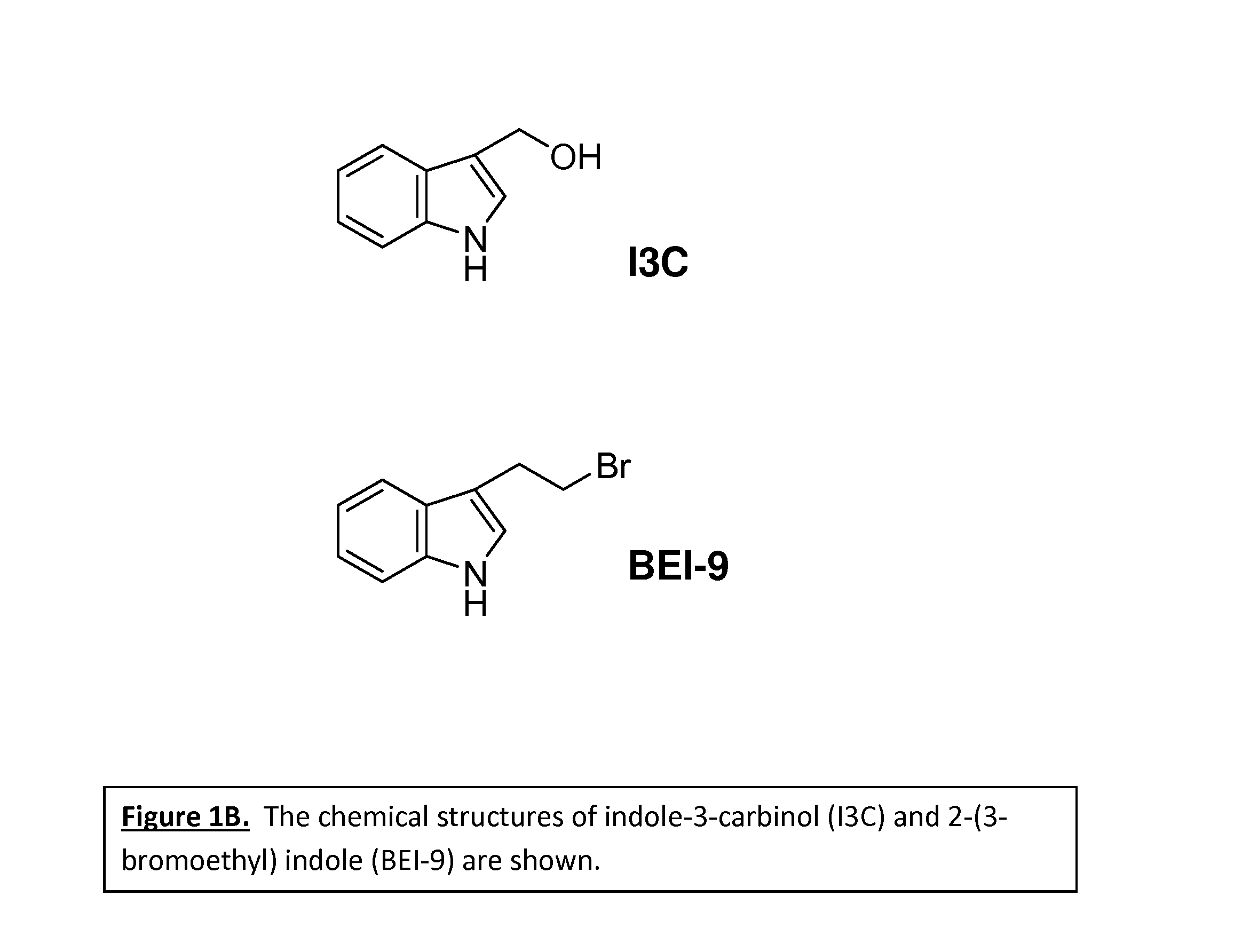
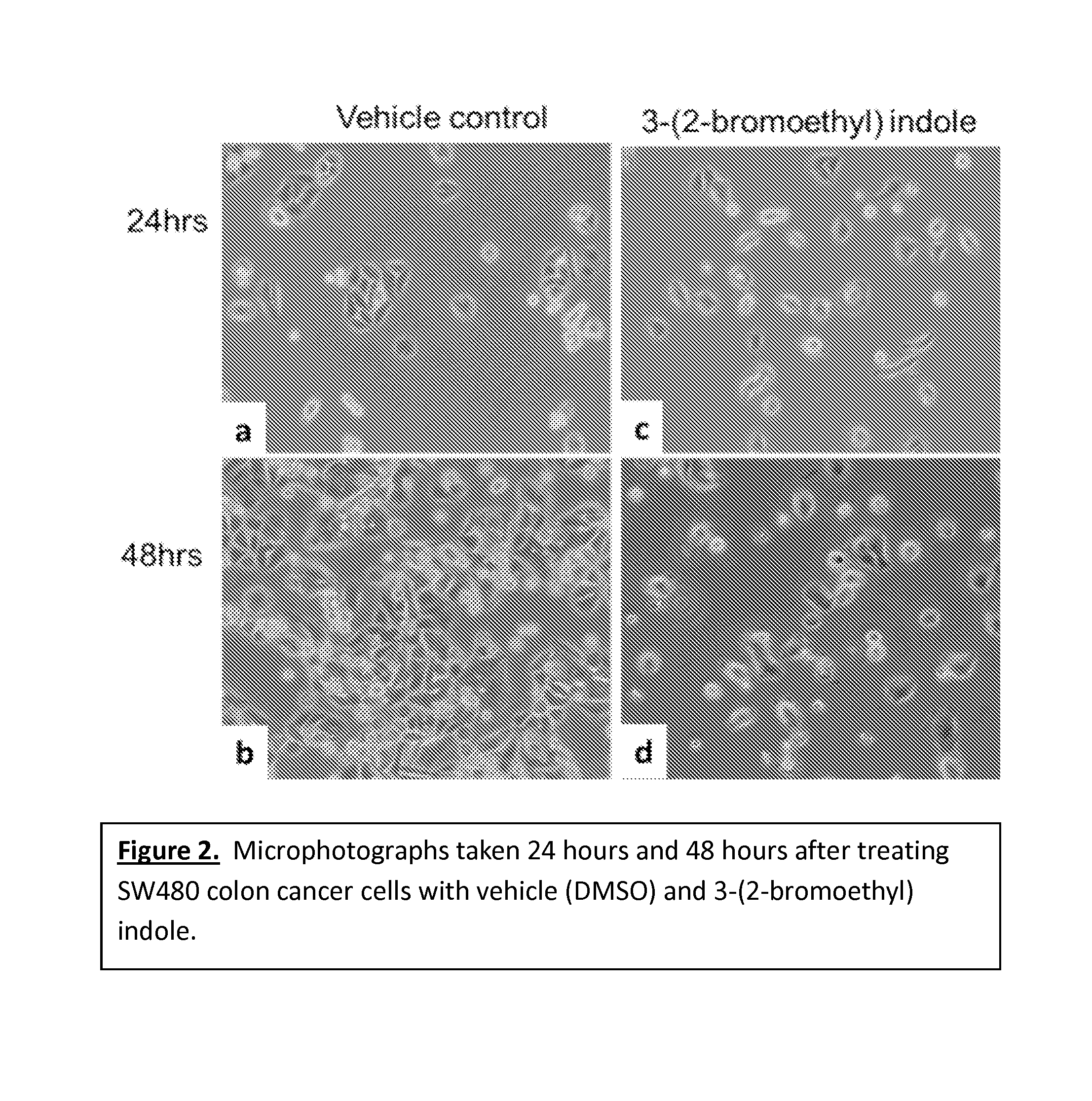
Anticancer treatment methods involving analogs and derivatives of 3-(2-substituted-ethyl) indole compounds
ActiveUS20150164860A1Organic active ingredientsOrganic chemistryPerylene derivativesCancer cell proliferation
Methods for inhibiting cancer cell proliferation and killing cancer cells are disclosed. Such methods comprise treating cancer cells with an indole compound having the structure of formula (I):wherein R is defined herein.
Owner:TUSKEGEE UNIVERSITY
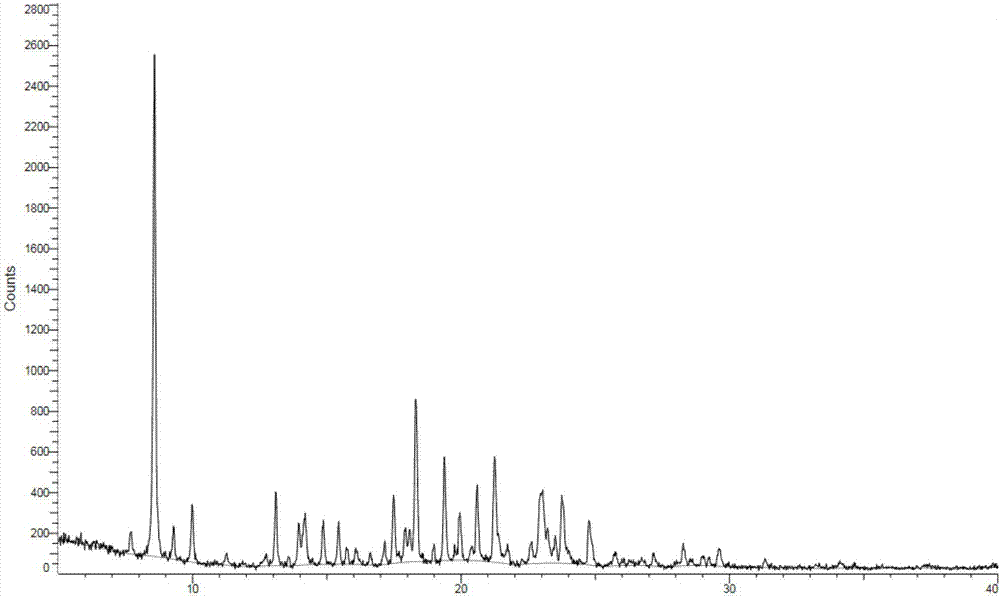
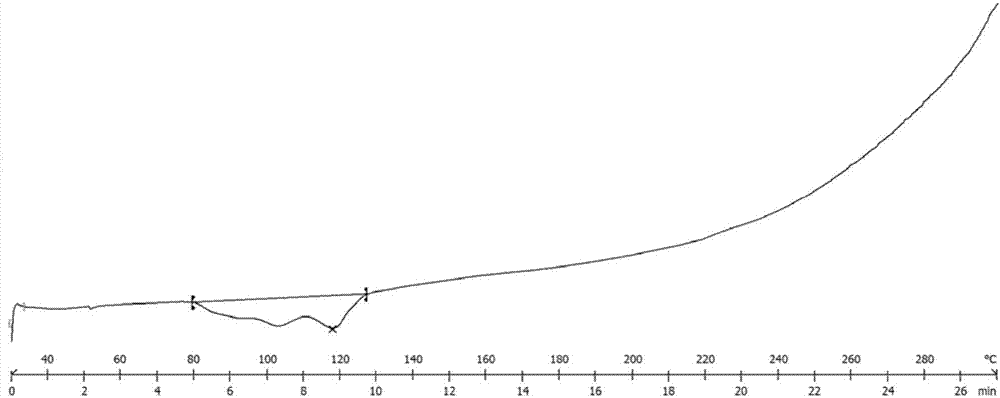
Crystals of anilinopyrimidine compound with indole substituted by trifluoroethyl group and salts thereof
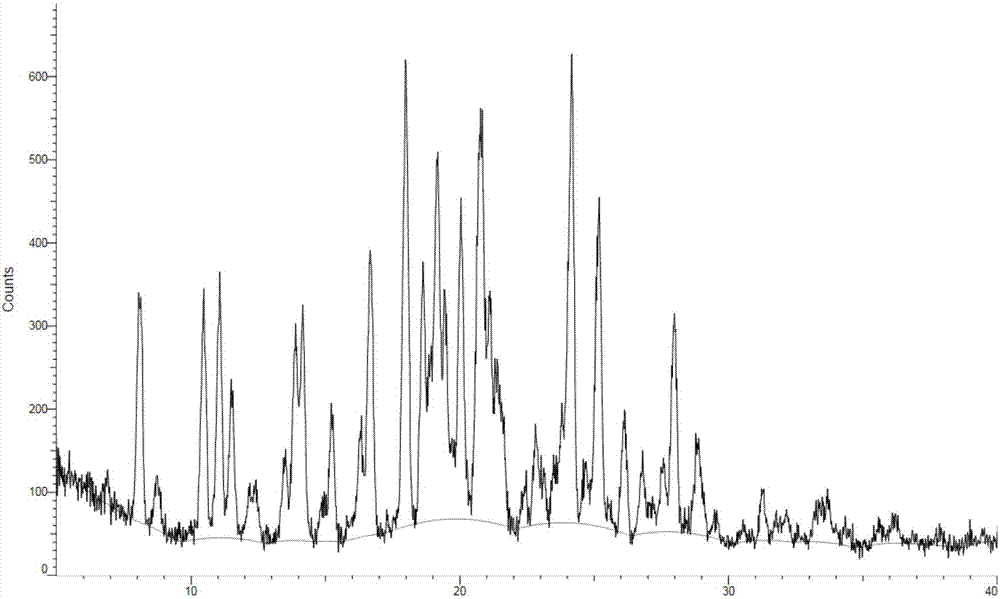
ActiveCN107973782AHigh purityHigh crystallinityOrganic active ingredientsOrganic chemistry methodsEGFR inhibitorsBis indole
The invention provides crystals of an anilinopyrimidine compound with indole substituted by a trifluoroethyl group and salts thereof, wherein the anilinopyrimidine compound is used as an EGFR inhibitor. Specifically, the invention relates to a crystal of the compound as shown in a formula I which is described in the specification, a crystal of a monomesylate of the compound as shown in the formulaI or a dimesylate of the compound as shown in the formula I, and further relates to preparation methods for the crystals, crystal compositions containing the crystals, and pharmaceutical compositionscontaining the crystals or the crystal compositions and medical application thereof. The crystals provided by the invention have the advantages of high purity, high crystallization degree, good stability, etc.
Owner:INVENTISBIO CO LTD +1
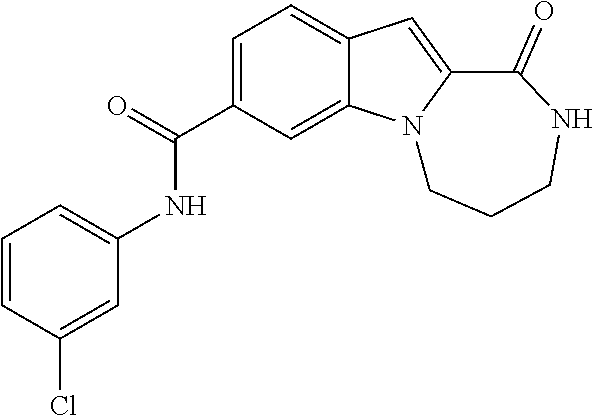
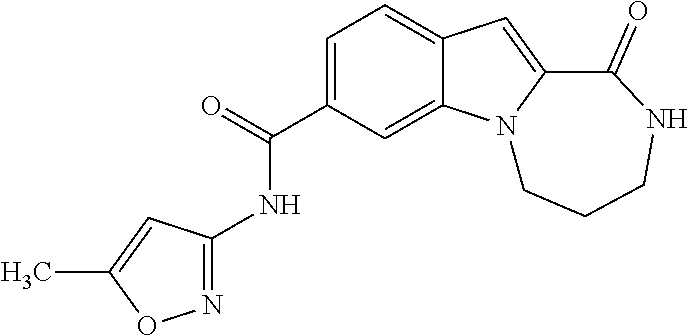
Heterocyclic compounds containing an indole core
Owner:BOEHRINGER INGELHEIM INT GMBH
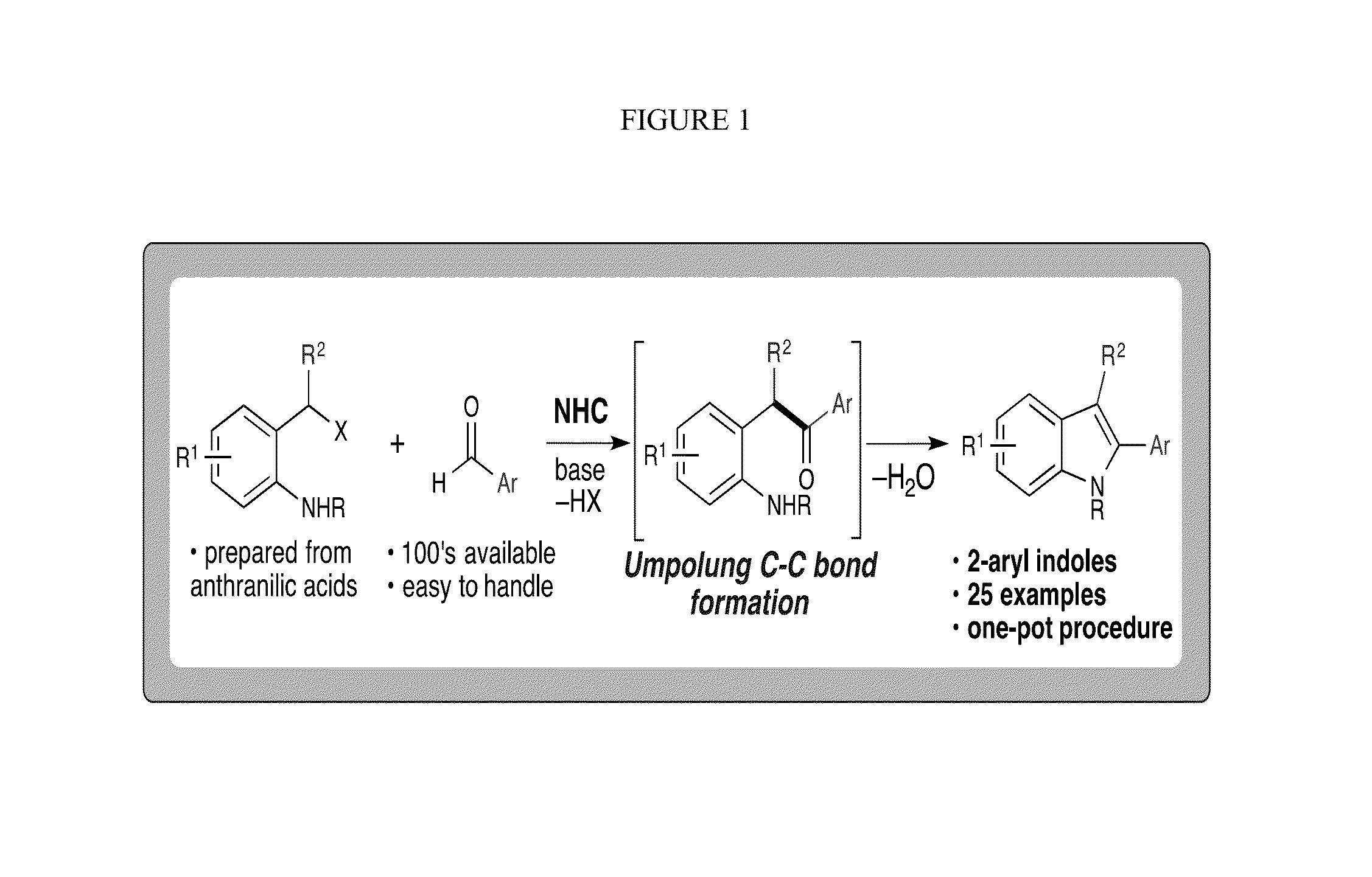
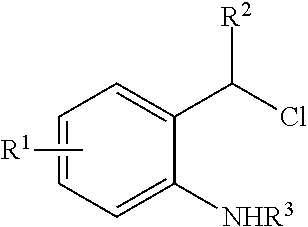
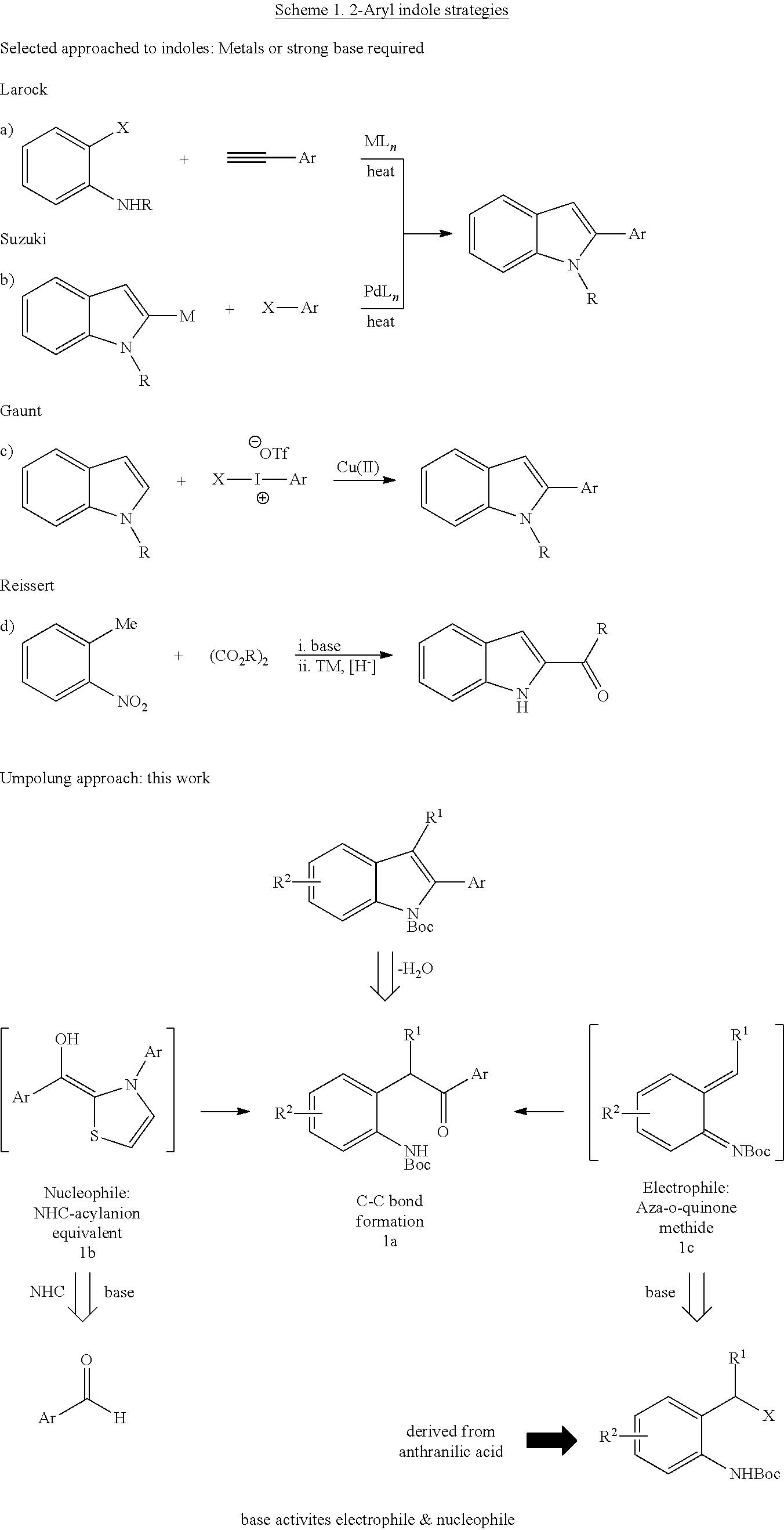
N-Heterocyclic Carbene-Catalyzed Synthesis of 2-Aryl Indoles
Owner:NORTHWESTERN UNIV
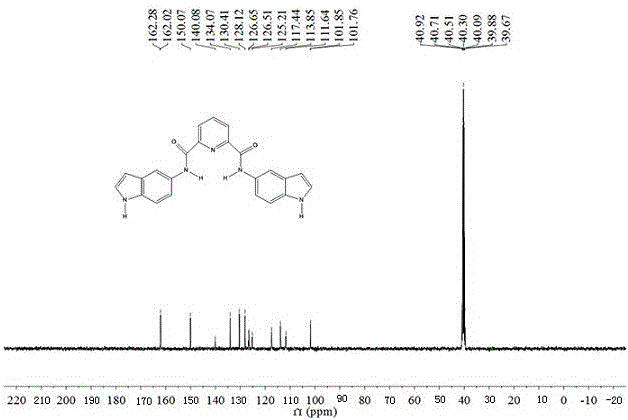
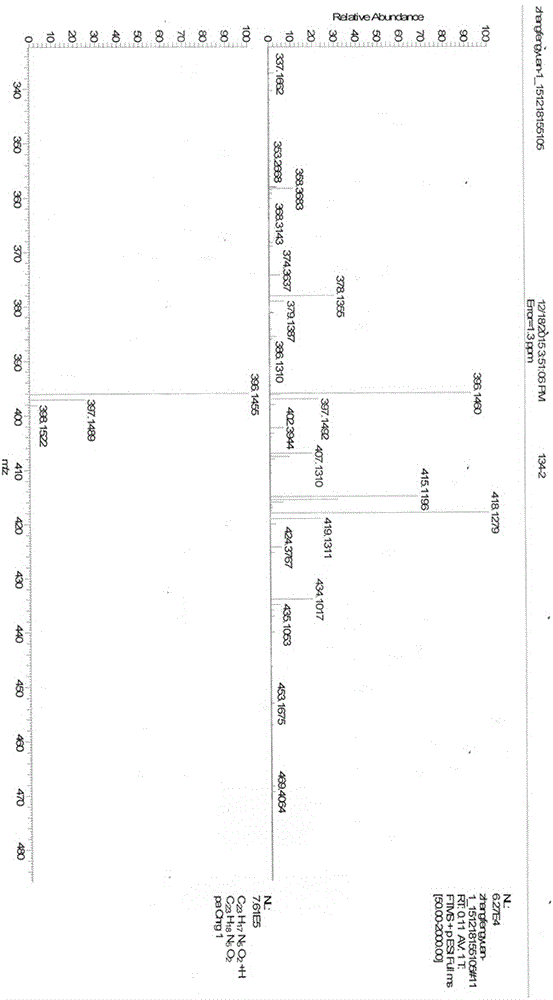
Synthesis method of ultraviolet fluorescence molecule probe, and nitrite ion detection of probe
ActiveCN106596542AEasy to synthesizeMild conditionsOrganic chemistryMaterial analysis by observing effect on chemical indicatorNitrite ionOptical property
The invention relates to a synthesis method of an ultraviolet fluorescence molecule probe N,N'-5-diindole-2,6-pyridinedicarboxamide, and an application of the probe in detection of nitrite ions in various practical samples. Synthesis of a double-arm cave-shaped compound adopting bisindole as a fluorescence group and pyridinedicarboxamide as a support realizes specific identification of the nitrite ions. The probe has the characteristics of stable optical properties, good specificity, high sensitivity, easiness in preparation, and low cost, and can detect the nitrite ions under a mild pH value (2-5) condition. When the N,N'-5-diindole-2,6-pyridinedicarboxamide is used to detect the content of the nitrite ions in the various practical samples, the existence or not of the nitrites in a sample can be qualitatively judged according to the color change, and the content of the nitrite ions in the sample can be detected through ultraviolet fluorescence spectrum.
Owner:LANZHOU UNIVERSITY +1
2,2-di(1H-indole-3-yl)-2H-acenaphthene-1-ketone compound and preparation method thereof
ActiveCN103936648ANo pollutionMild reaction conditionsOrganic chemistryChemical synthesisPtru catalyst
The invention relates to a 2,2-di(1H-indole-3-yl)-2H-acenaphthene-1-ketone compound and a preparation method thereof, and belongs to the technical field of organic chemical synthesis. The preparation method comprises the following steps: reacting in an anhydrous alcohol solvent in the presence of a sulfonic acid compound by taking acenaphthequinone and an indole compound as raw materials, cooling a reaction liquid to reach a room temperature after the reaction is completely carried out; adding distilled water into the reaction liquid so as to separate out yellow solid powder; carrying out suction filtration on the yellow solid powder and washing the yellow solid powder with absolute ethyl alcohol; and carrying out vacuum drying so as to obtain the 2,2-di(1H-indole-3-yl)-2H-acenaphthene-1-ketone compound. The method carried out by taking the anhydrous alcohol as a solvent and the sulfonic acid compound as a catalyst is simple and easy in raw material obtainment, low equipment requirement, moderate in reaction condition, good in catalytic effect as well as simple and convenient to operate. The yield of the obtained compound can reach above 97%; and the purity of the obtained compound can reach above 99%. The 2,2-di(1H-indole-3-yl)-2H-acenaphthene-1-ketone compound, the structure of which is authenticated by the nuclear magnetic resonance, is suitable for industrial production.
Owner:山东金鹏德盛斋扒鸡有限公司
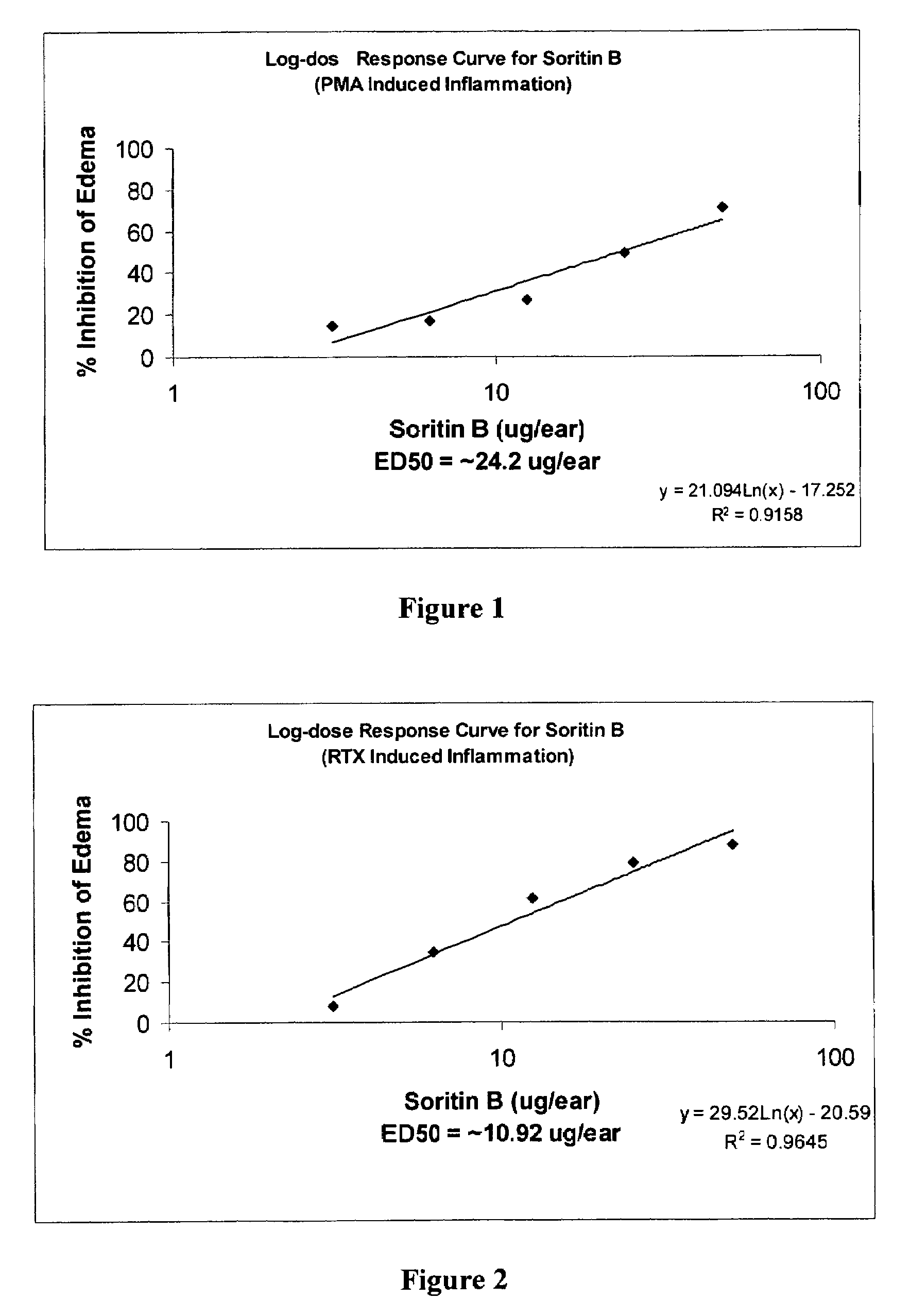
Method for the synthesis of soritin compounds
Disclosed herein are methods for synthesizing biologically active bis-heterocyclic compounds, e.g. bis-indoles. In particular, methods for making Soritin compounds such as Soritin B, bis-(1H-indol-3-yl)-acetic acid methyl ester, Soritin C, bis-2,2-(1-methyl-indol-3-yl) acetic acid methyl ester, Soritin D, bis-2,2-(1-methyl-indol-3-yl) acetic acid and Soritin compositions comprising the Soritin compounds are disclosed. Also disclosed are pharmaceutical formulations comprising the Soritin compounds and Soritin compositions.
Owner:RGT UNIV OF CALIFORNIA
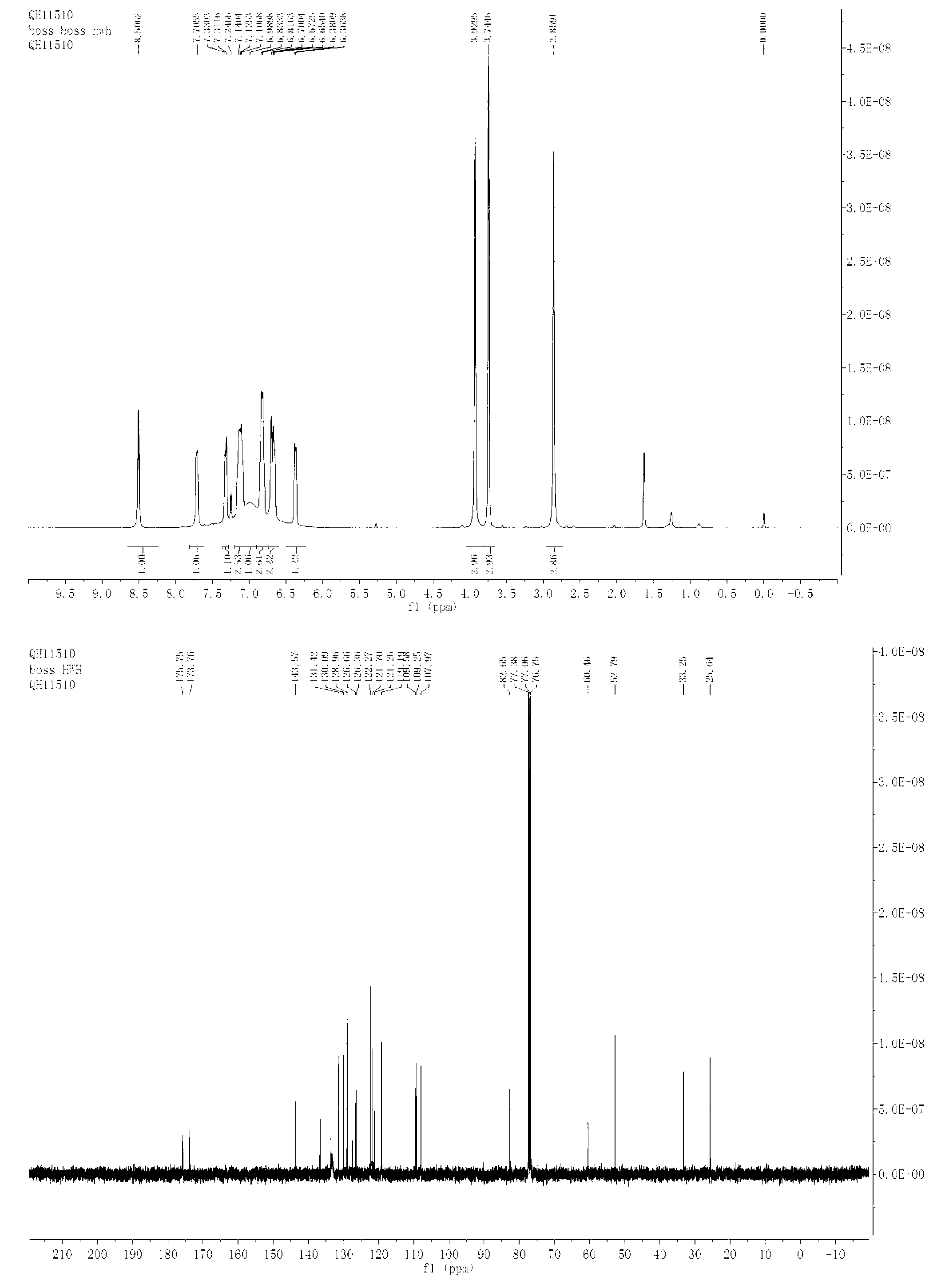
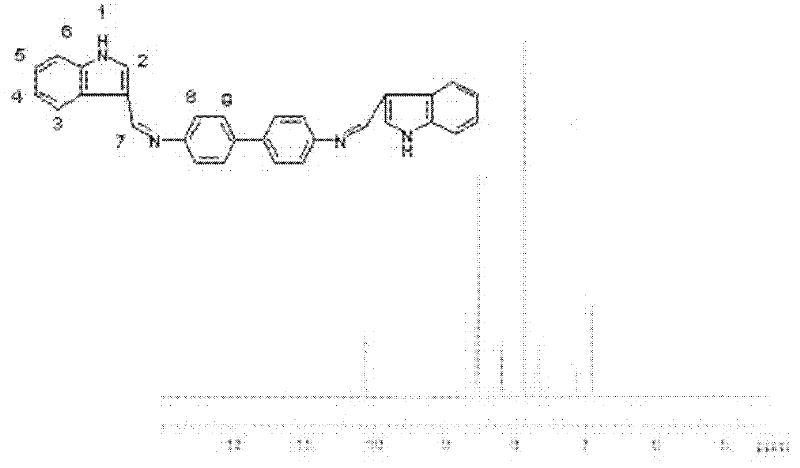
Bis-Schiff bases synthesized by condensing indole-3-carboxaldehyde and pyridine diamine and preparation method thereof
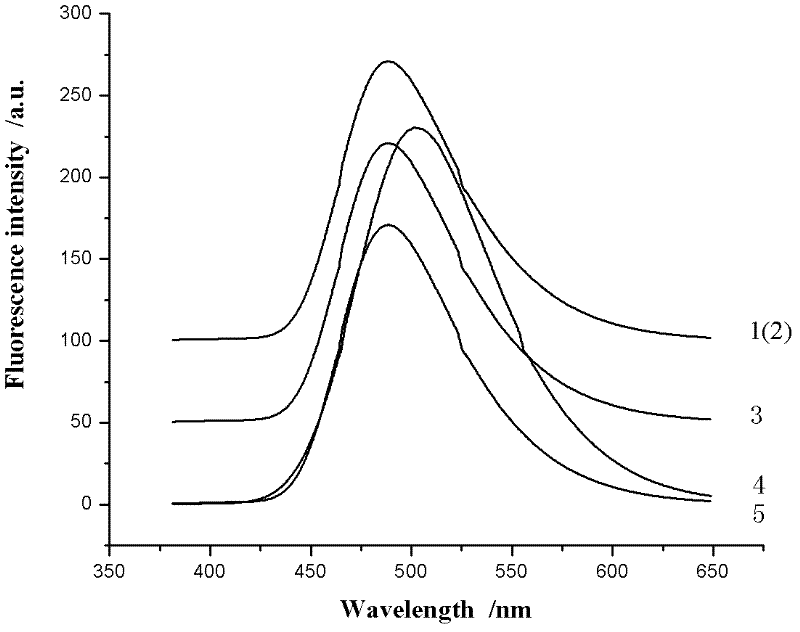
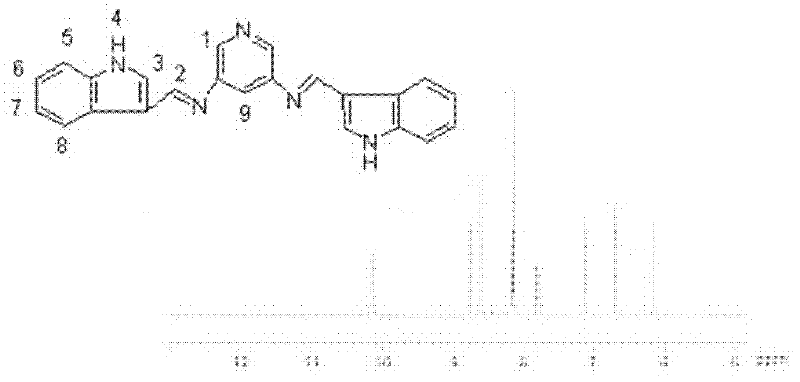
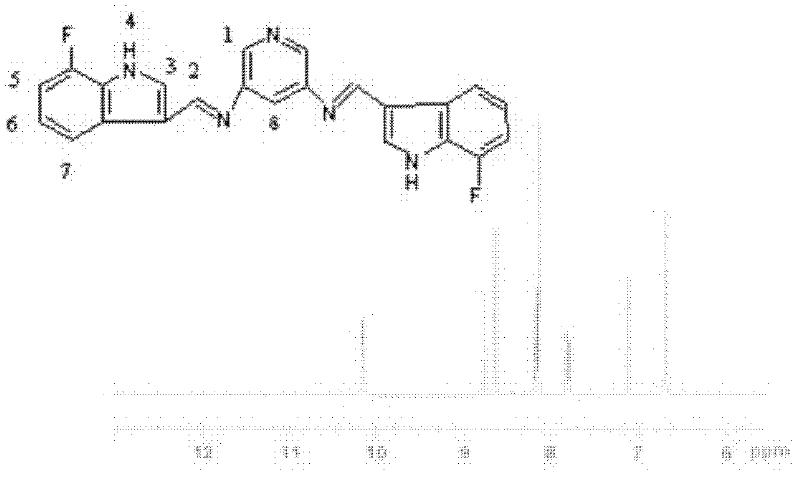
InactiveCN102584796AGood antibacterial effectAntibacterial agentsOrganic chemistryFluorescenceAntibacterial agent
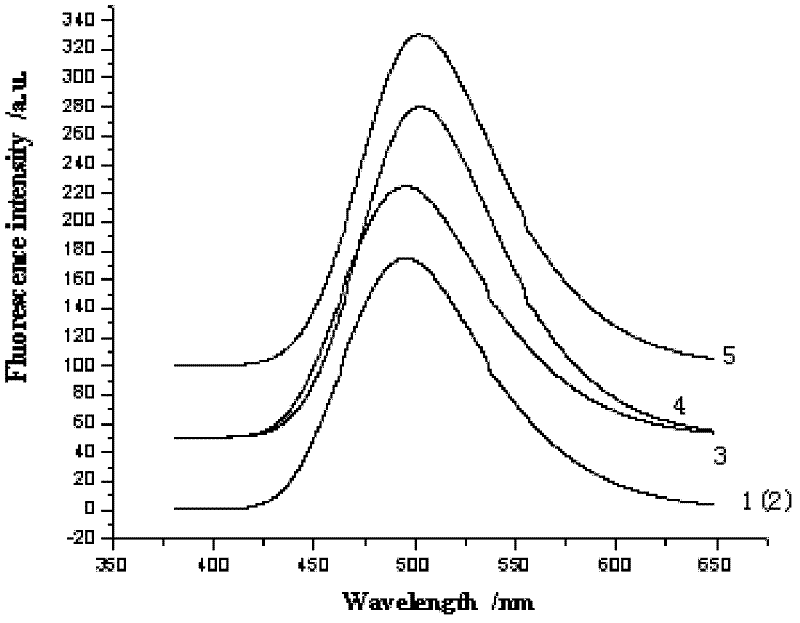
The invention relates to bis-Schiff bases synthesized by condensing indole-3-carboxaldehyde and pyridine diamine and a preparation method thereof. A series of indole Schiff bases with conjugate structure are obtained by taking 3,5-pyridine diamine and indole-3-carboxaldehyde and derivatives thereof as the raw materials and adopting condensation reaction between aldehyde group and primary amine. The bis-Schiff bases and the preparation method have the following beneficial effects: the synthesis cost is low; the yield is high; the reaction conditions are mild; the products are easy to purify; the compounds have good bioactivity and can be applied to the field of medicines as antibacterial agents; and with conjugate and rigid plane structures, the compounds also show stronger fluorescence and can be applied to the field of materials as fluorescent materials.
Owner:QILU UNIV OF TECH
Bisindole alkaloid derivative, and synthesis method and application thereof
InactiveCN103254112AEfficient constructionHigh selectivityOrganic chemistryAntiparasitic agentsOrganic solventSynthesis methods
The invention discloses a synthesis method of a bisindole alkaloid derivative. A diazo compound, an indole derivative and isatin derivative are used as raw materials, rhodium acetate is used as a catalyst, an organic solvent is used as a solvent, and the raw materials, the catalyst and the solvent are reacted in one step at the temperature of minus 10 DEG C to 50 DEG C to obtain the indole alkaloid derivative. The synthesis method has the advantages of high-efficiency atom economy, high selectivity, high yield, low consumption of the catalyst, simplicity and safety in operation and the like. The bisindole alkaloid derivative can be used as an active molecule precursor to be widely applied in the medical chemical field.
Owner:EAST CHINA NORMAL UNIV
Bis-Schiff bases synthesized by condensing indole-3-carboxaldehyde and benzidine and preparation method thereof
InactiveCN102584671AGood antibacterial effectStrong fluorescenceAntibacterial agentsOrganic chemistryAntibacterial agentBis indole
The invention relates to bis-Schiff bases synthesized by condensing indole-3-carboxaldehyde and benzidine and a preparation method thereof. A series of indole Schiff bases with a conjugate structure are obtained by taking benzidine and indole-3-carboxaldehyde and derivatives thereof as the raw materials and adopting condensation reaction between aldehyde group and primary amine. The bis-Schiff bases and the preparation method have the following beneficial effects: the synthesis cost is low; the yield is high; the reaction conditions are mild; the products are easy to purify; the compounds have good bioactivity and can be applied to the field of medicines as antibacterial agents; and with conjugate and rigid plane structures, the compounds also show stronger fluorescence and can be appliedto the field of materials as fluorescent materials.
Owner:QILU UNIV OF TECH
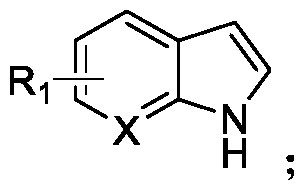
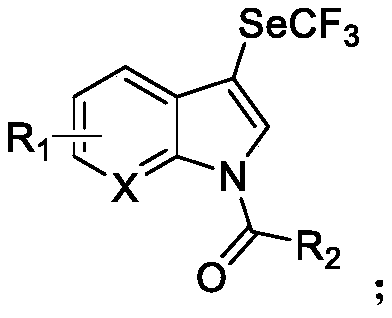
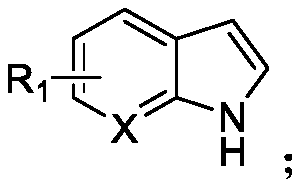
N-acylated trifluoro-methylseleno bifunctionalization method for indole derivative
InactiveCN110372565AHigh yieldRealization of N-acylated trifluoromethaneselenoyl difunctionalizationOrganic chemistrySolventAromatic hydrocarbon
The invention discloses an N-acylated trifluoro-methylseleno bifunctionalization method for an indole derivative. The method comprises the following steps: dissolving a trifluoro-methylseleno ammoniumsalt or a metal salt and an oxidant into a solvent, adding an indole derivative one droplet by one droplet, performing a stirring reaction, adding water for quenching after the reaction is completed,and performing column chromatography, so as to obtain an N-acylated trifluoro-methylseleno product, wherein the oxidant is peroxy-acyl. The method provided by the invention is simple and convenient to operate, gentle in condition, easy in raw material obtaining and high in product yield, in addition, the trifluoro-methylseleno ammonium salt or the mtal salt with nucleophilicity is adopted as a trifluoro-methylseleno source, and N-acylated trifluoro-methylseleno bifunctionalization of electron-rich aromatic hydrocarbon (the indole derivative) is achieved for a first time.
Owner:WUHAN UNIV OF TECH
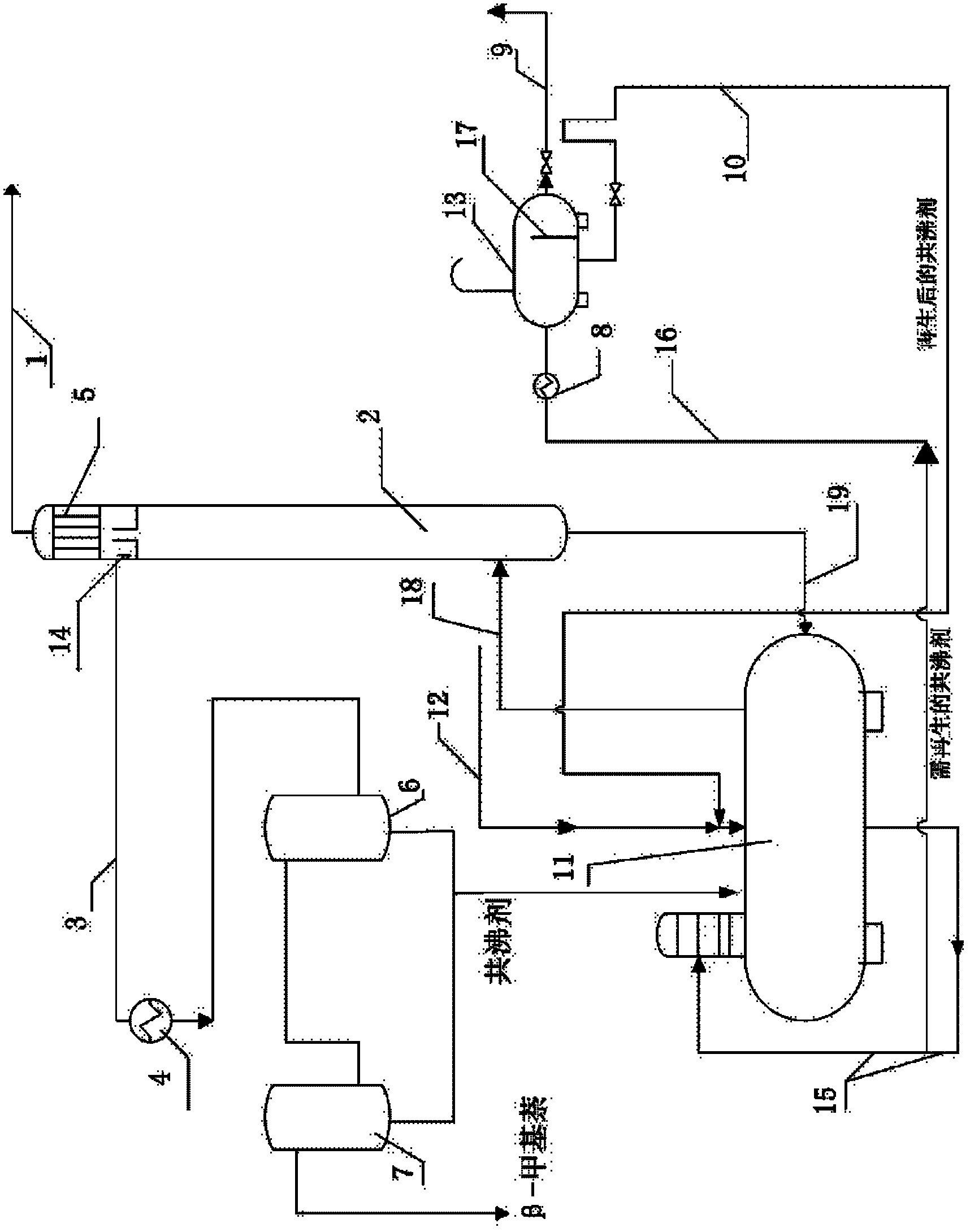
Method and device for removing indole from beta-methylnaphthalene
ActiveCN102935292BImprove difficult situationsMeet export demandDistillation purification/separationDistillation separationChemical industryReflux
Relating to the field of chemical industry, the invention discloses a method and a device for removing indole from beta-methylnaphthalene. Structurally, the device comprises a distillation kettle and azeotropic distillation tower. An overflow recovery baffle is disposed at a tower top recovery outlet of the azeotropic distillation tower. A tower top recovery pipeline is connected to a cooler, a separation tank A and a separation tank B in order. The method includes: adding a mixture of the raw material beta-methylnaphthalene and an azeotropic agent into the distillation kettle, controlling the temperature of the kettle bottom at 145-147DEG C; maintaining the azeotropic distillation tower at a pressure of -70-90kpa and the tower top at a temperature of 140-142DEG C; carrying out intermittent azeotropic distillation separation, subjecting the evaporated liquid to cooling by the tower top cooler and internal reflux; and subjecting the azeotropic distillation tower top recovered liquid to cooling by the cooler, then leaving the liquid to flow into the separation tank A and the separation tank B in order, performing standing layering, and recovering the product. By means of the method and the device, after distillation separation, the content of the raw material beta-methylnaphthalene can be increased to over 98%, and the indole content decreased to an undetectable degree. The device and the method provided in the invention are simple and are easy to operate.
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
Synthetic method for tetracyclic indole skeletons under catalysis of protonic acid
ActiveCN109384794AEasy to prepareReduce usageOrganic chemistryP-Toluenesulfonic acidEthane Dichloride
The invention belongs to the technical field of medicines, and provides a method for synthesizing tetracyclic indole alkaloid skeletons through tandem reactions. The method has a reaction formula which is described in the specification. With the method provided by the invention, reaction substrates are indole-ynamides with different substituents; a catalyst is selected from the group consisting ofcamphorsulfonic acid (CSA), diphenyl phosphate (DPP), p-toluenesulfonic acid (TsOH), p-nitrobenzenesulfonic acid (NsOH) and bistrifluoromethanesulfonimide (HNTf2); a medium needed in the reactions isselected from the group consisting of dichloromethane, dichloroethane, chloroform, toluene, acetonitrile, tetrahydrofuran and acetone; the reactions can be performed under stirring at a low temperature or room temperature; and through the reactions, a series of tetracyclic indole skeleton-containing compounds can be directly produced through tandem cyclization of the indole-ynamide substrates inthe presence of protonic acid. The method provided by the invention has the characteristics of simple operation, wide application range, few by-products, high yield, green reaction, etc.
Owner:SHENYANG PHARMA UNIVERSITY
Method and device for removing indole from beta-methylnaphthalene
ActiveCN102935292ASimple processEasy to operateDistillation purification/separationDistillation separationChemical industryTower
Relating to the field of chemical industry, the invention discloses a method and a device for removing indole from beta-methylnaphthalene. Structurally, the device comprises a distillation kettle and azeotropic distillation tower. An overflow recovery baffle is disposed at a tower top recovery outlet of the azeotropic distillation tower. A tower top recovery pipeline is connected to a cooler, a separation tank A and a separation tank B in order. The method includes: adding a mixture of the raw material beta-methylnaphthalene and an azeotropic agent into the distillation kettle, controlling the temperature of the kettle bottom at 145-147DEG C; maintaining the azeotropic distillation tower at a pressure of -70-90kpa and the tower top at a temperature of 140-142DEG C; carrying out intermittent azeotropic distillation separation, subjecting the evaporated liquid to cooling by the tower top cooler and internal reflux; and subjecting the azeotropic distillation tower top recovered liquid to cooling by the cooler, then leaving the liquid to flow into the separation tank A and the separation tank B in order, performing standing layering, and recovering the product. By means of the method and the device, after distillation separation, the content of the raw material beta-methylnaphthalene can be increased to over 98%, and the indole content decreased to an undetectable degree. The device and the method provided in the invention are simple and are easy to operate.
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
Tanshinone framework spliced bisindole or bispyrrole compound as well as preparation method and application thereof
ActiveCN107188924ASynthesis is cheap and easy to getImprove compatibilitySteroidsAntineoplastic agentsAlkaneSolvent
The invention discloses a tanshinone framework spliced bisindole or bispyrrole compound. The tanshinone framework spliced bisindole or bispyrrole compound is prepared by adding substituted indole or substituted pyrrole and tanshinone, according to the mol ratio of 3 to 1, into a polar solvent acetonitrile, and carrying out addition reaction for 72h under the condition of heating at 50 DEG C. A framework contains a multi-bioactivity tanshinone framework and a bisindole or bispyrrole alkane framework, and can provide a compound source for bioactivity screening; the tanshinone framework spliced bisindole or bispyrrole compound has important application value in screening of multi-target multipurpose drugs and a pharmaceutical industry. The tanshinone framework spliced bisindole or bispyrrole compound has the advantages that the operation is simple and feasible; the synthesis of raw materials is cheap and easy to realize, and can be carried out in various polar organic solvents; the tanshinone framework spliced bisindole or bispyrrole compound also has relatively good air stability and wide applicability, and has good compatibility on various substituent groups; the compound has a potential of being developed into an anti-tumor drug.
Owner:GUIZHOU UNIV
Method for preparing bi-indolyl fluorene derivative
The invention relates to a method for preparing a bi-indolyl fluorene derivative. According to the method, fluorenone and indole are taken as reactants and are dissolved in a solvent so as to react at 25DEG C-100DEG C to obtain the bi-indolyl fluorene derivative, wherein acid is taken as a catalyst, the molar ratio of fluorenone, indole and catalyst is 1:(1-4):(0.05-0.2). According to the method, the raw materials and the catalyst are cheap and are easy to obtain, and the reaction conditions are mild; the bi-indolyl fluorene derivative is built with one step through cascade reaction, the method has few operation steps, is convenient to separate and purify, and has high synthesis efficiency; and the method also can be used for preparing other large conjugated system molecules with applied research significance through reaction of aromatic heterocycle, benzene ring and fluorenone.
Owner:山东禾雅生物科技有限公司
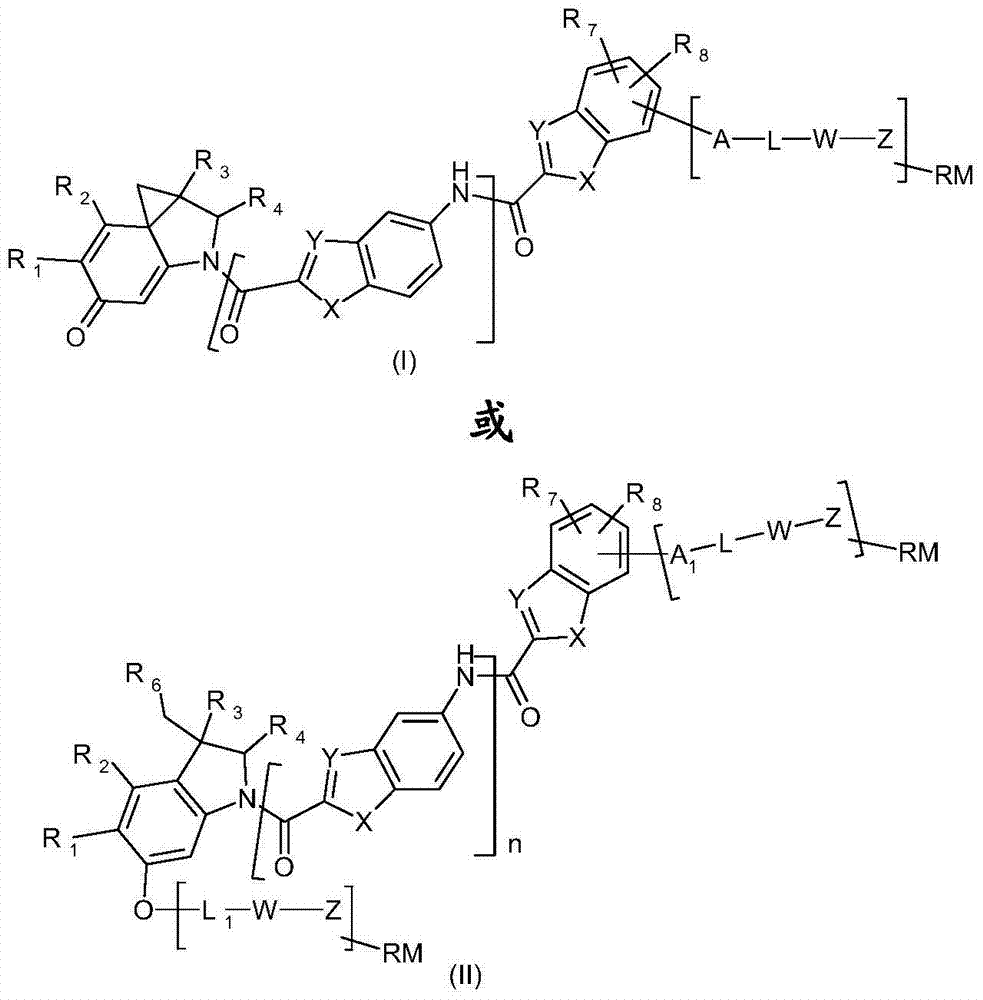
Functionalized thieno-indole derivatives for the treatment of cancer
The invention relates to new functionalized thieno-indole derivatives of formula (I) or (II) which have cytotoxic activity and are useful in treating diseases such as cancer and cellular proliferation disorders. The invention also relates to the use of these functionalized thieno-indole derivatives in the preparation of conjugates. Formula (I) or (II) wherein R1 and R2 taken together form a group (D) or (G): wherein R5 is hydrogen or C1-C4 alkyl; R3 and R4 are independently hydrogen, C1-C4 alkyl or C1-C4 hydroxyalkyi; n is 0, 1 or 2; each of X is independently -O-, -S- or -NR4-; each of Y is independently -CH = or -N=; R7 and R8 are independently hydrogen, halogen, hydroxy, C1-C4 alkoxy, cyano, -NHCOOR3, -C(NH)NH2 or -NR3R4; A is -O-, -NH- or -CO-; L is null or a conditionally-cleavable moiety; W is null or a self-immolative moiety comprising one or more self-immolative groups; Z is null or a peptidic, non peptidic or hybrid peptidic and non peptidic linker; RM is null or a reactive moiety; R6 is a leaving group; A1 is null or A; L1 is hydrogen or L.
Owner:NERVIANO MEDICAL SERVICES SRL
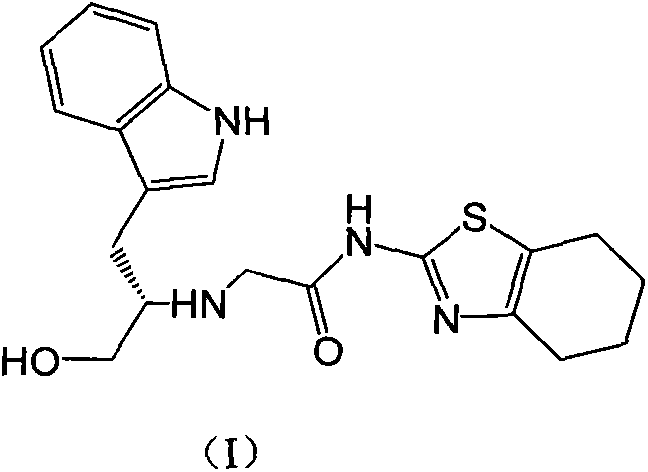
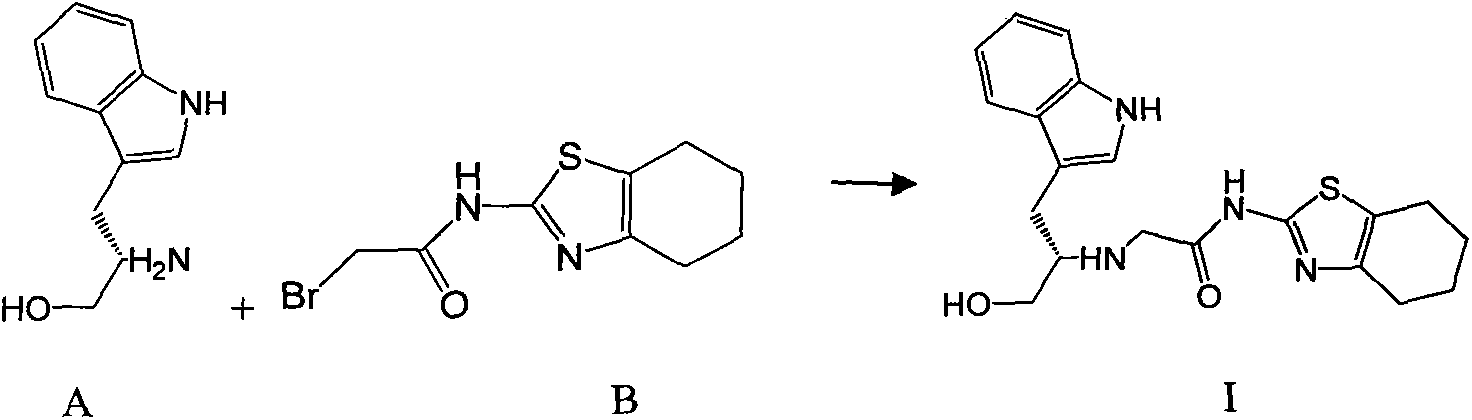
Indole-substituted thiazolo cyclohexane compound and antineoplastic applications thereof
InactiveCN103408541AOrganic active ingredientsOrganic chemistryPerylene derivativesAntitumor activity
Owner:ZHEJIANG PHARMA COLLEGE
Ervatamia plant extract as well as extraction and separation method and application thereof
ActiveCN113248525APrevent proliferationInhibit hypertrophyNervous disorderOrganic chemistry methodsRight ventricular hypertrophyBlood vessel
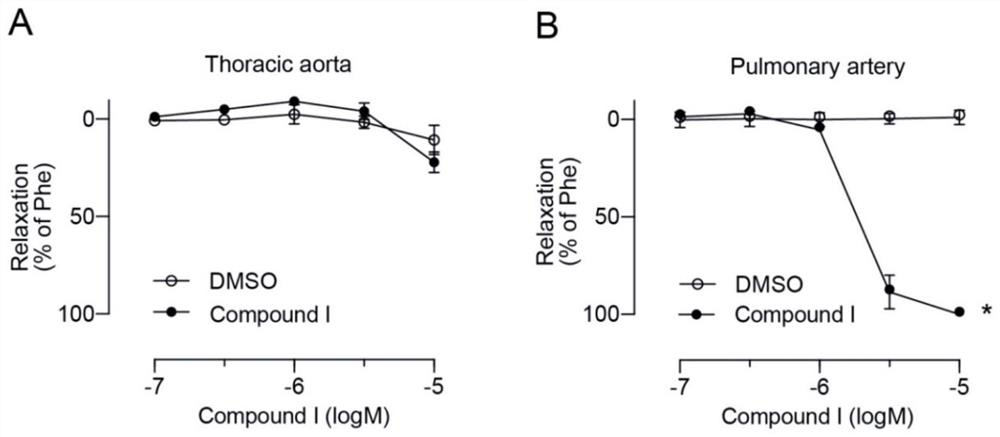
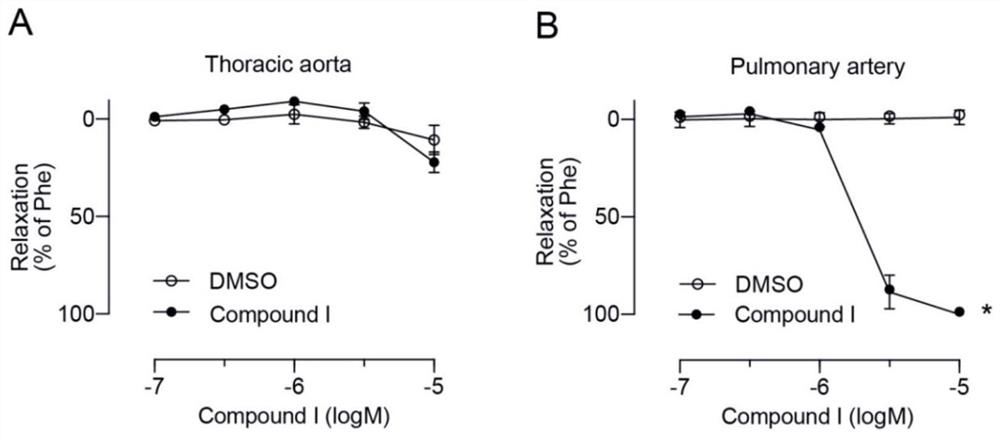
The invention discloses an ervatamia plant extract as well as an extraction and separation method and application thereof. The ervatamia plant extract contains one or more than one bisindole alkaloid selected from bisindole alkaloids E-1 to E-16. The ervatamia plant extract obtained through extraction and separation contains bisindole alkaloid compounds, and can selectively relax pulmonary arteries, inhibit proliferation of pulmonary artery endothelial cells and vascular smooth muscle cells, reduce right ventricular diastolic pressure of pulmonary arterial hypertension mice and inhibit right ventricular hypertrophy. In addition, the series of bisindole alkaloid compounds have chemical structure types different from chemical structure of existing targeted drugs for treating pulmonary arterial hypertension, and the ervatamia plant extract is expected to be developed into novel targeted drugs for treating pulmonary arterial hypertension.
Owner:JINAN UNIVERSITY
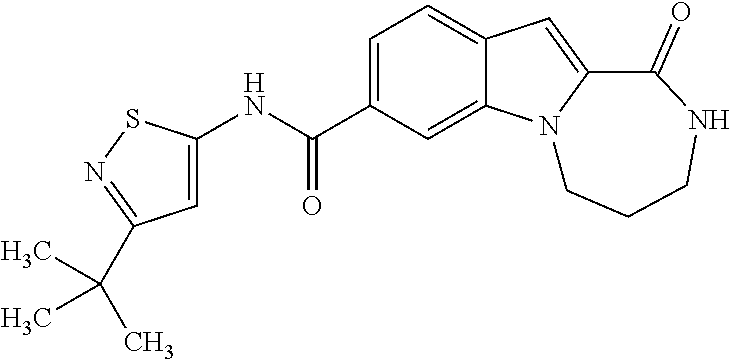
Heterocyclic compounds containing an indole core
Owner:BOEHRINGER INGELHEIM INT GMBH
Bisindole alkaloid compound as well as synthesis method and application thereof
ActiveCN113248524APrevent proliferationInhibit hypertrophyNervous disorderOrganic chemistry methodsRight ventricular hypertrophyPharmacology
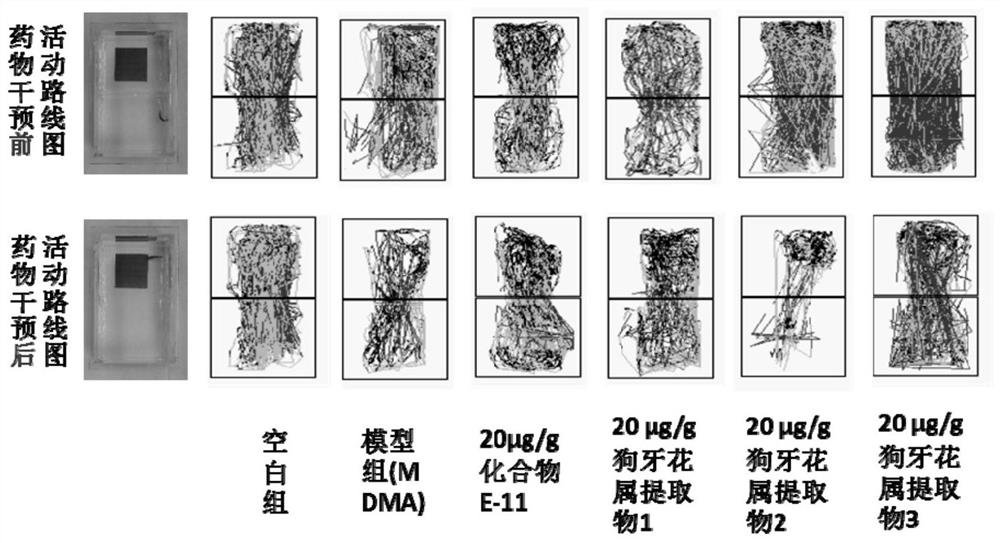
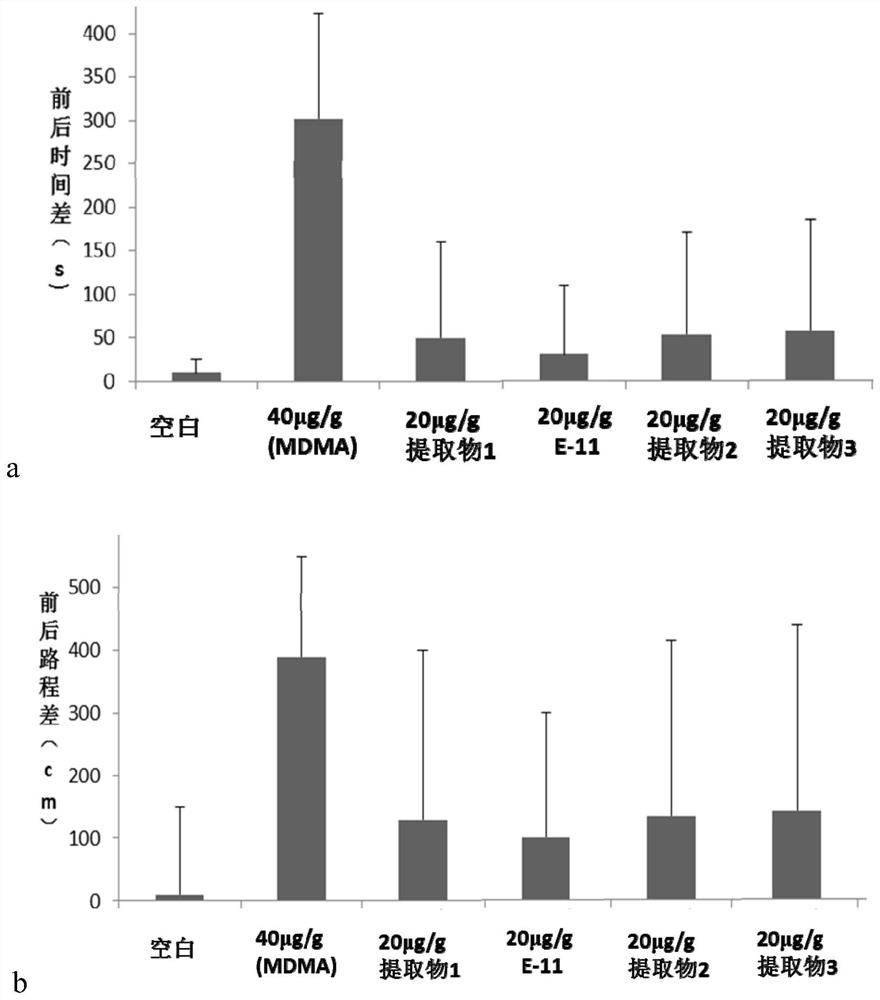
The invention discloses a bisindole alkaloid compound as well as a synthesis method and application thereof. The compound has a structure as shown in formula I. In the formula I, R1 is independently selected from C1-C4 alkoxy or hydrogen; if R1 is alkoxy, n is 1 or 2; R2 is independently selected from C1-C4 alkyl groups or hydrogen; R3 is independently selected from a group consisting of C1-C4 alkoxy carbonyl groups or hydrogen; R4 is hydrogen; R5 is independently selected from a C1-C4 alkyl group or a C1-C4 hydroxyalkyl group; and R6 is independently selected from carbonyl, hydroxyl or hydrogen. The bisindole alkaloid compound can selectively relax pulmonary artery, inhibit proliferation of pulmonary artery endothelial cells and vascular smooth muscle cells, reduce right ventricular diastolic pressure of mice with pulmonary arterial hypertension and inhibit right ventricular hypertrophy. The bisindole alkaloid compound can resist drug addiction in a dose-dependent manner, has a chemical structure type different from that of an existing anti-addiction drug, and is expected to be developed into a novel anti-addiction drug.
Owner:JINAN UNIVERSITY
Synthesis method of nitrogenous medicine intermediate indole derivative
InactiveCN106866489AHigh yieldGood application prospectOrganic chemistryChemical synthesisSynthesis methods
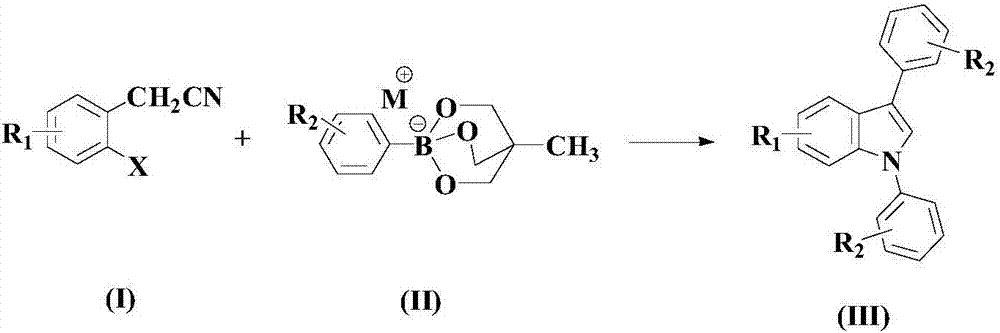
The invention relates to a synthesis method of an indole derivative shown by a nitrogenous medicine intermediate (III). The method comprises the following steps of in a solvent and in the insert gas atmosphere, under the existence of catalysts, nitrogenous ligands and acid promoters, a compound shown by a formula (I) and a compound shown by a formula (II) take a reaction; after the reaction is completed, post treatment is performed, so that a compound shown by the formula (III) is obtained. The formulas (I), (II) and (III) are shown as the accompanying drawing, wherein the R1 is selected from H or halogen; the R2 is selected from H, C1-C6 alkyls, C1-C6 alkoxy or halogen; or R2 and the substituted phenyl jointly form naphthyl. The method has the advantages that through the comprehensive selection and cooperation of the proper substrates, catalysts, nitrogenous ligands, acid promoters, solvents and the like, the range of the substrates is expanded; the aryl-substituted indol compound can be obtained at a good yield, so that the good application prospects and study values are realized in the field of organic chemical synthesis. The fire-new method is provided for the synthesis of the compounds of the kind.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Synthetic method of trifluoromethyl-containing spiro indoline or acetal
PendingCN109232606AGood economic environmentHigh selectivityOrganic chemistry methodsFuranTrifluoromethylation
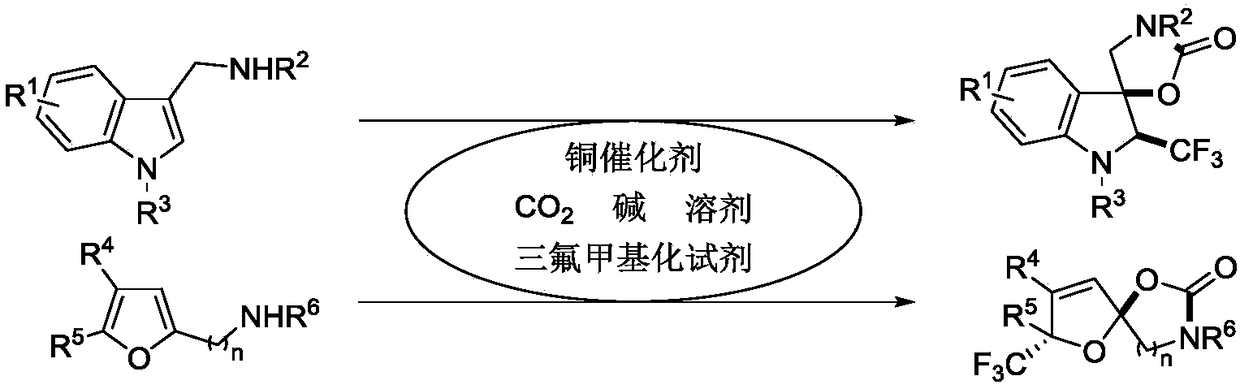
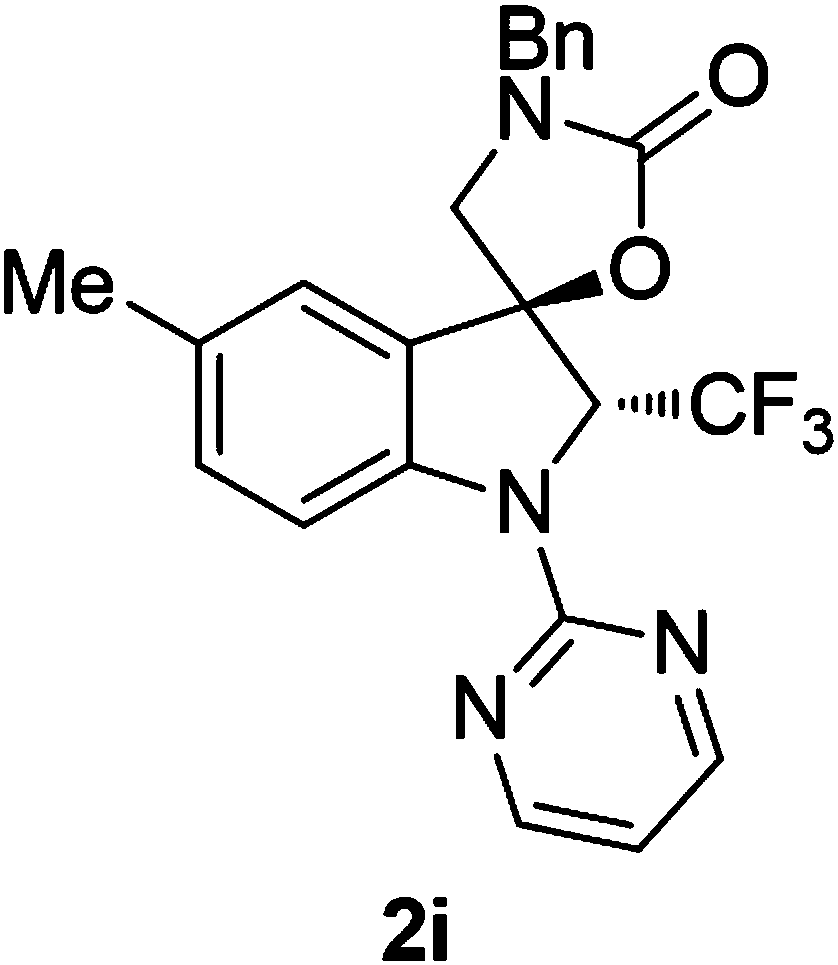
The invention provides a synthetic method of trifluoromethyl-containing spiro indoline or acetal. The synthetic method comprises the steps as follows: a substituted indole substrate or a substituted furan substrate reacts in a solvent in the presence of CO2, a copper catalyst, alkali and a trifluoro methylation reagent to obtain the trifluoromethyl-containing spiro indoline or trifluoromethyl-containing spiro acetal. A trifluoromethyl-containing spiro indoline and spiro acetal compound is constructed under redox neutral and mild conditions, and besides, greenhouse gas CO2 is utilized in the synthetic method, so that the synthetic method has better economic and environment significance. The obtained trifluoromethyl-containing spiro indoline and spiro acetal compound has high chemical areasand diastereoisomer selectivity and has the advantages of better functional group compatibility, wider substrate range and high probability of gram-grade scale synthesis.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole](https://images-eureka.patsnap.com/patent_img/88432790-d6fe-44cc-a995-27f6bb71773c/US08426850-20130423-D00000.png)
![Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole](https://images-eureka.patsnap.com/patent_img/88432790-d6fe-44cc-a995-27f6bb71773c/US08426850-20130423-D00001.png)
![Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole Semiconducting component including 1-[3-aryl-isoindolyl-(1)-imino]-3-aryl-IH-isoindole](https://images-eureka.patsnap.com/patent_img/88432790-d6fe-44cc-a995-27f6bb71773c/US08426850-20130423-D00002.png)