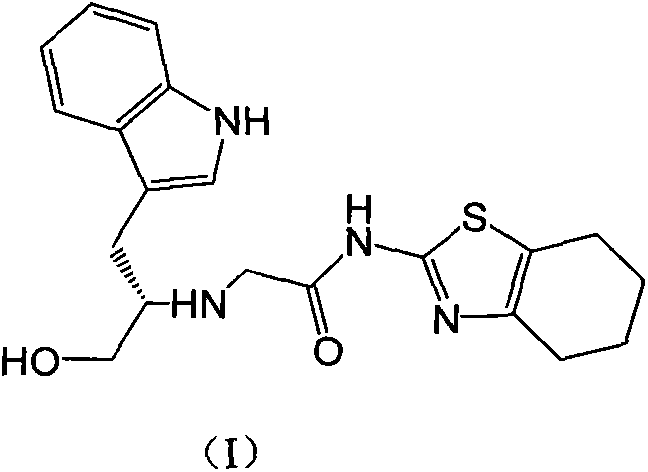
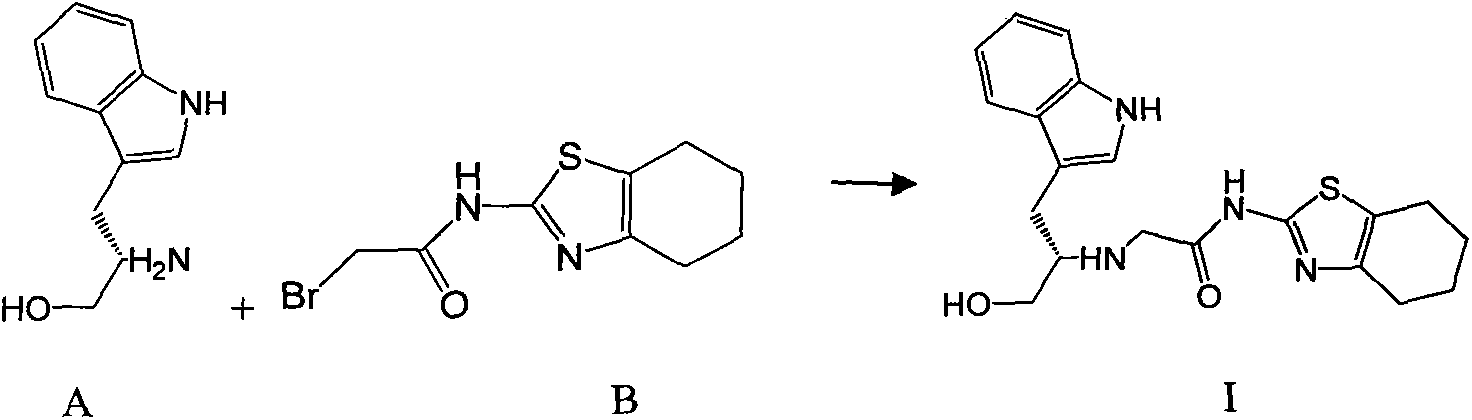
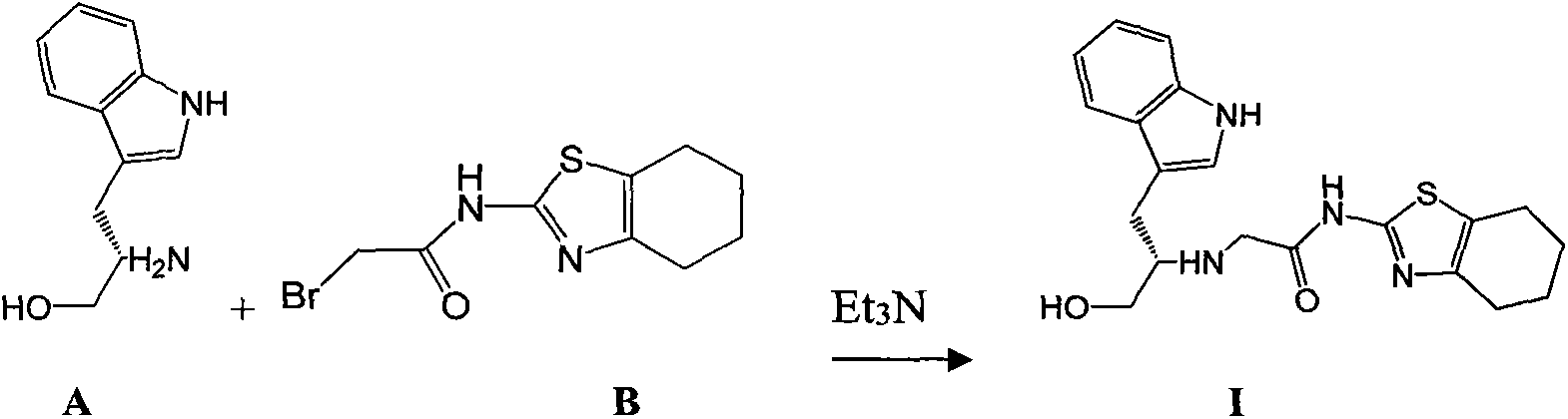
Indole-substituted thiazolo cyclohexane compound and antineoplastic applications thereof
A compound and anti-tumor drug technology, which is applied in the application field of thiazolocyclohexane derivatives and anti-tumor, and can solve the problems of cancer multidrug resistance, poor selectivity, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Reaction raw materials: commercially available or self-made.
[0022] 1.90g (10mmol) of compound A and 2.75g (10mmol) of compound B were dissolved in 20mL of dry THF, and then 3.04g (30mmol) of Et 3 N, then stirred at room temperature until the reaction was complete (24-48 hours). The reaction mixture was poured into ice water, extracted with 50 mL×3 dichloromethane, the combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was evaporated on a rotary evaporator to obtain a crude product of I, which was purified by column chromatography. The pure product of I was obtained as a white solid, mp.157-159°C; MS, m / z=385 ([M+H] + ).
Embodiment 2
[0024]
[0025] 1.90 (10 mmol) of compound A and 2.75 g (10 mmol) of compound B were dissolved in 20 mL of dry THF, and 3.88 g (30 mmol) of DIPEA were added, followed by reflux with stirring until the reaction was complete (within 10 hours). The reaction mixture was poured into ice water, extracted with 50 mL×3 dichloromethane, the combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was evaporated on a rotary evaporator to obtain a crude product of I, which was purified by column chromatography. The pure product of I was obtained as a white solid, mp.157-159°C; MS, m / z=385 ([M+H] + ).
Embodiment 3
[0027] (1) Material
[0028] Cell lines: leukemia HL-60 cells, gastric adenocarcinoma SGC-7901 cells, breast cancer MCF-7 cells, and lung cancer A-549 cells were all purchased from Shanghai Cell Institute, Chinese Academy of Sciences.
[0029] Reagents: MTT, Amresco aliquots; DMEM medium, Gibco; calf serum, Lanzhou Minhai Biology; trypsin, Amresco aliquots; fluorouracil injection, 0.25g / 10ml (branch), Tianjin Jinyao Amino Acid Co., Ltd.
[0030] Instrument: ultra-clean bench, Suzhou Purification Equipment Factory; CO 2 Incubator, Thermo Company, model: HERA Cell150; inverted microscope, Carl Zeiss Company, model: Axiovert200; enzyme-linked immunoassay instrument, TECAN Company, model: Sunrise; centrifuge, Kerdro Company, model: Heraeus.
[0031] (2) method
[0032] Cell culture: Tumor cells were inoculated in DMEM medium containing 10% calf serum, 100IU / ml penicillin G sodium salt and 100ug / ml streptomycin sulfate, placed at 37°C, 100% relative humidity, containing 5% CO 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com