Bis-Schiff bases synthesized by condensing indole-3-carboxaldehyde and pyridine diamine and preparation method thereof
A technology of formaldehyde pyridine diamine bis-Schiff base and pyridine diamine, which is applied in the field of indole-3-formaldehyde pyridine diamine bis-Schiff base and its preparation, and can solve the problem of indole bis-Schiff base synthesis. Related reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
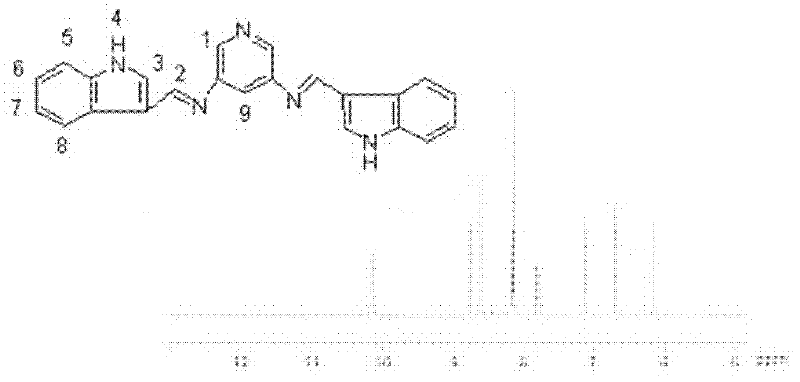
[0032] Weigh 1.09g (0.01mol) of pyridine-3,5-diamine, weigh 2.89g (0.02mol) of indole-3-carbaldehyde, dissolve in 50ml of tetrahydrofuran, and add it to a 100ml container equipped with a thermometer and a stirring device. In a four-neck bottle. Stirring was started, the reaction temperature was controlled at 65°C, and the constant temperature was reacted for 3 hours. The solvent was distilled off under reduced pressure to obtain a white solid powder, which was recrystallized with ethanol and dried in vacuum at 50°C for 6 hours to obtain the target product: indole-3-formaldehyde condensate Pyridine-3,5-diamine bis-Schiff base.
[0033] Elemental Analysis: C 23 h 17 N 5 : %C: 76.01 (75.99); %N 19.27 (19.17); %H 4.71 (4.84) (measured values in brackets).
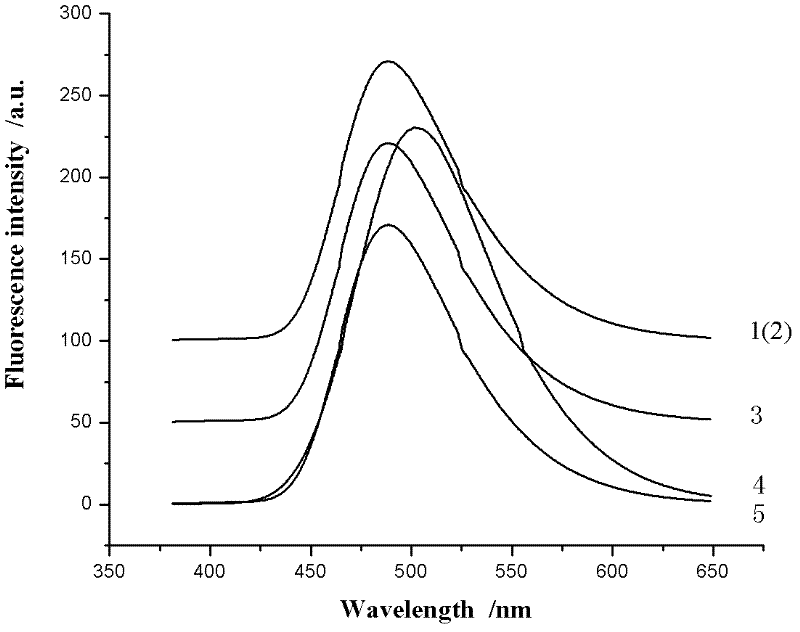
[0034] NMR analysis (NMR spectrum see figure 2 ):
[0035] Table 1 was obtained by analyzing the structural formula and proton nuclear magnetic resonance spectrum of compound 1. Compound 1 has nine kinds of hydrogen ...
Embodiment 2
[0040] Weigh 1.09g (0.01mol) of pyridine-3,5-diamine, weigh 2.89g (0.02mol) of indole-3-carboxaldehyde, dissolve in 50ml of acetone, and add to 100ml of acetone equipped with a thermometer and a stirring device. In a four-neck bottle. Stirring was started, the reaction temperature was controlled at 65°C, and the reaction was carried out at constant temperature for 3 hours. The solvent was distilled off under reduced pressure to obtain a light yellow solid powder, which was recrystallized with ethanol and dried in vacuum at 50°C for 6 hours to obtain the target product: indole-3-carbaldehyde Pyridine-3,5-diamine bis-Schiff base.
[0041] Elemental Analysis: C 23 h 17 N 5 : %C: 76.01 (75.89); %N19.27 (19.19); %H4.71 (4.92) (measured values in brackets).
[0042] NMR analysis (NMR spectrum see figure 2 ): The analysis result is the same as that of compound 1.
Embodiment 3
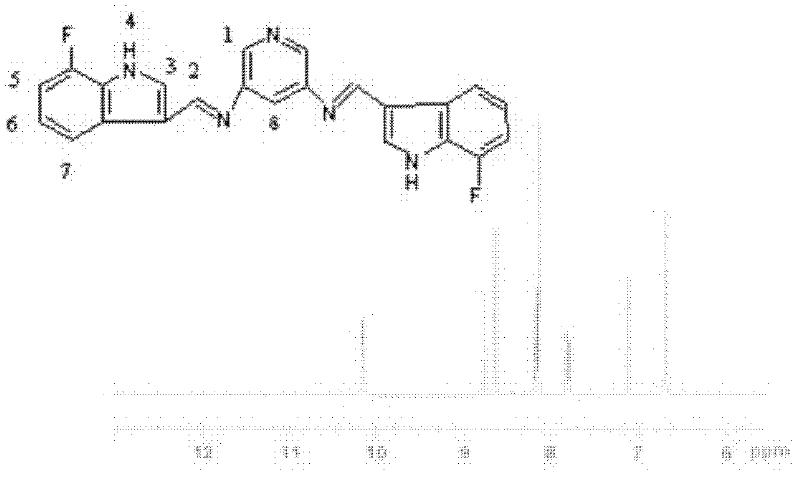
[0044] Weigh 1.09g (0.01mol) of pyridine-3,5-diamine, weigh 6.75g (0.05mol) of 7-fluoroindole-3-carbaldehyde, dissolve in 50ml of 1,4-dioxane, add To a 100ml four-necked bottle equipped with a thermometer and a stirring device. Stirring was started, the reaction temperature was controlled at 65°C, and the constant temperature reaction was carried out for 12 hours. The solvent was distilled off under reduced pressure to obtain a yellow solid powder, which was recrystallized with ethanol and dried in vacuum at 50°C for 6 hours to obtain the target product: 7-fluoroindole-3 -Formaldehyde p-phenylenediamine bis-Schiff base.
[0045] Elemental Analysis: C 23 h 15 N 5 f 2 : %C: 69.17 (70.00); %H: 3.79 (3.89); %N: 17.53 (17.43); %F: 9.51 (8.68) (measured values in brackets).
[0046] NMR analysis (NMR spectrum see image 3 ):
[0047]Table 2 was obtained by analyzing the structural formula and proton nuclear magnetic resonance spectrum of compound 2. Compound 2 has eight ki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com